Metallurgical Abstracts on Light Metals and Alloys vol.57
Hydrogen storage and ignition properties of pelletized catalyzed magnesium
Shigehito Isobe and Kentaro Shibata
Faculty of Engineering, Hokkaido University
[Published in International Journal of Hydrogen Energy 53 (2024) 517-521]
https://doi.org/10.1016/j.ijhydene.2023.12.032
E-mail: isobe[at]eng.hokudai.ac.jp
Key Words: Hydrogen storage, Catalyzed magnesium, Pelletizing
Catalyzed magnesium (MgH2+1mol%Nb2O5) can quickly absorb hydrogen even at room temperature, but there is a risk of ignition due to reaction with oxygen when handled in the atmosphere. In this study, we attempted to suppress ignition by pelletizing catalyzed magnesium powder. In addition, the effect of pelletizing conditions on the hydrogen storage properties of catalyzed magnesium was investigated. The pellets were formed at a forming pressure from 140 MPa to 1400 MPa. The higher the pelletizing pressure, the more the particles adhered to each other, and it was confirmed that an adhered layer of about 5 μm was formed on the pellet surface. This is thought to have reduced the pathway for gas molecules to penetrate from the pellet surface to the interior, resulting in a decrease in the hydrogen absorption rate and ignitability.
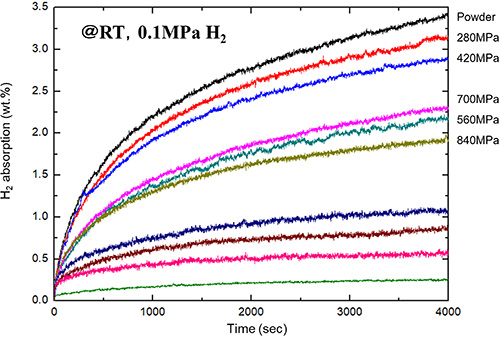
Hydrogen absorption curves of catalyzed Mg as powder and pellets at room temperature under 0.5 MPa of H2 gas.
