Metallurgical Abstracts on Light Metals and Alloys vol.57
Generation of aluminum hydroxide from industrial waste
Saori Hayashiguchi* and Koji Maekawa*
* National Institute of Technology, Kitakyushu College
[Published in proceedings of the 2024 Annual Meeting IEE of Japan, Vol. 4-163 (2024), pp. 272]
E-mail: maekawa[at]apps.kct.ac.jp
Key Words: Aluminum hydroxide, Baemite, Hydrogen generation
Hydrogen generation conditions for industrial use of aluminum hydroxide, which is produced as a byproduct of hydrogen generation reactions, were investigated experimentally. Powders of by-products obtained under the existing experimental conditions of hydrogen generation were quantitatively analyzed using an XRD analyzer. It was found that about 60% of the by-product was aluminum hydroxide, about 8% was unreacted aluminum, and about 20% was baemite (AlOOH), which is a type of aluminum hydroxide but has a different crystal form. Based on these results, the addition of sodium hydroxide and the setting of the reaction temperature were examined. As a result, it was confirmed that the purity of aluminum hydroxide was improved by suppressing the formation of baemite and decreasing the ratio of unreacted aluminum compared to existing methods.
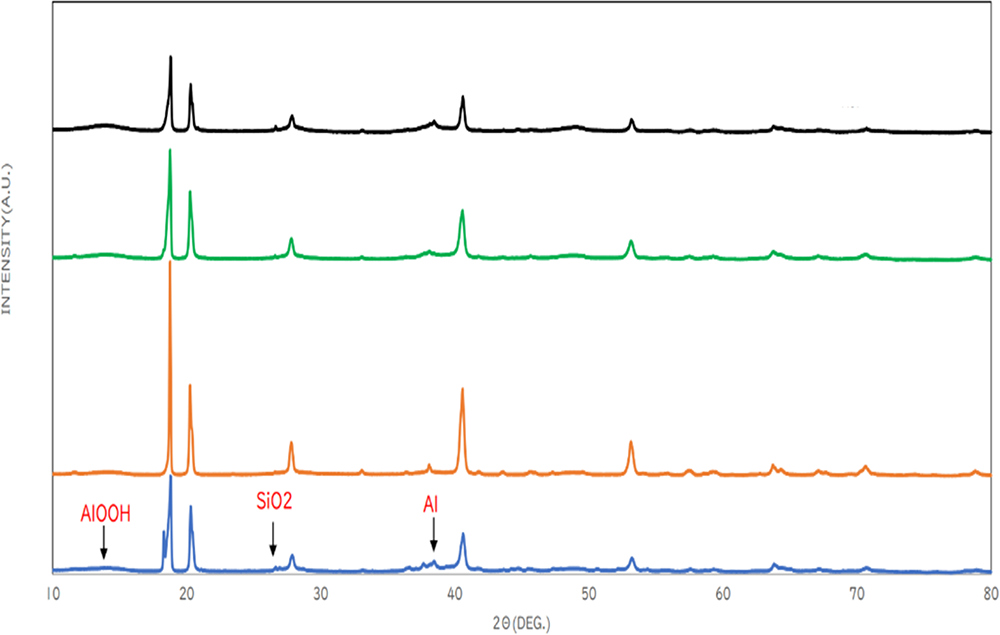
XRD analysis showed that the sample that was reacted with the addition of a catalyst and a long reaction time with a small temperature change was the most effective in suppressing the formation of baemite.
