Metallurgical Abstracts on Light Metals and Alloys vol.57
Difference in the Precursory Process of the Intergranular Corrosion of Aged Al-Cu and Al-Cu-Mg Alloys in 0.1 M NaCl
Hiroki Yoshida*, **, Masashi Nishimoto*, Izumi Muto*, Mai Takaya***, Yoshihiko Kyo***, Tadashi Minoda*** and Yu Sugawara**
* Department of Materials Science, Tohoku University
** Present address: Research & Development Division, UACJ Corporation
*** Research & Development Division, UACJ Corporation
[Published in Journal of The Electrochemical Society, Vol. 170 (2023), 111501]
https://doi.org/10.1149/1945-7111/ad0666
E-mail: mutoi[at]material.tohoku.ac.jp
Key Words: Al-Cu alloy, intergranular corrosion, pitting corrosion, intermetallics
Real-time in situ optical microscopy observations of the initiation behavior of intergranular corrosion on artificially aged Al-4.5Cu and Al-4.5Cu-1.5Mg were performed in naturally aerated 0.1 M NaCl at pH 6.0. For both alloys, the discoloration of intermetallic particles occurred before intergranular corrosion, and a discolored coarse intermetallic particle on the grain boundary acted as the initiation site for intergranular corrosion (Al2Cu for Al-4.5Cu and Al2CuMg for Al-4.5Cu-1.5Mg). The discoloration of Al2Cu particles was localized and occurred only on a small number of particles. However, almost all Al2CuMg particles were discolored; the overall surface of the particles was discolored uniformly. The discoloration of Al2Cu on Al-4.5Cu led to micropitting. In contrast, the discoloration of Al2CuMg on Al-4.5Cu-1.5Mg caused the trenching of particles. The difference in the initiation behavior of intergranular corrosion was discussed in terms of these precursory phenomena.
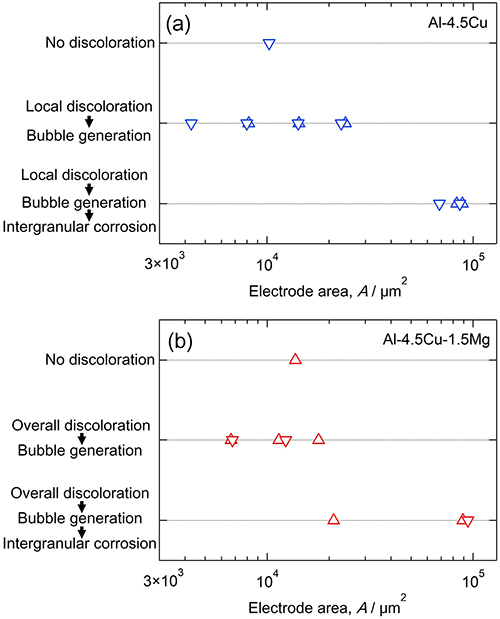
Effect of the size of the working electrode area on the occurrence of corrosion phenomena on (a) Al-4.5Cu and (b) Al-4.5Cu-1.5Mg alloys during 3.6-ks immersion in the naturally aerated 0.1 M NaCl (pH 6.0).
