Metallurgical Abstracts on Light Metals and Alloys vol.57
Formation of Hard Anodic Films on the 7075-T6 Aluminum Alloy by Anodization in Sulfuric Acid and Ethylene Glycol
Takuma Sano, Yuki Wakabayashi and Hidetaka Asoh
Department of Applied Chemistry, Kogakuin University
[Published in Surf. Coat. Technol., Vol. 459 (2023), 129399]
https://doi.org/10.1016/j.surfcoat.2023.129399
E-mail: asoh[at]cc.kogakuin.ac.jp
Key Words: Aluminum alloy, Anodization, Porous alumina, Film-growth efficiency, Film hardness
The 7075-T6 aluminum alloy was anodized in 1.5 mol·dm-3 sulfuric acid alone for 60 min, and small horizontal pores, along with the typical straight pores, were formed inside the pore wall, attributed to the chemical dissolution of the anodic film during the anodization. Conversely, after adding 50 vol% ethylene glycol to the sulfuric acid, the porous film almost maintained its initial structure with a low porosity even with the same anodization period of 60 min. The coating ratio and Martens hardness of the film formed in the mixture of sulfuric acid and ethylene glycol increased by 1.35-fold and 3.4-fold, respectively, compared with the reference values of the film formed in sulfuric acid alone. The properties of the film strongly reflect the dissolution behavior of not only the alloying elements in the anodic film but also the alumina, which constitutes the cell structure. The effect of the addition of alcohol on the hardness of the anodic films is universal and in good agreement with the previous results obtained with high-purity aluminum.
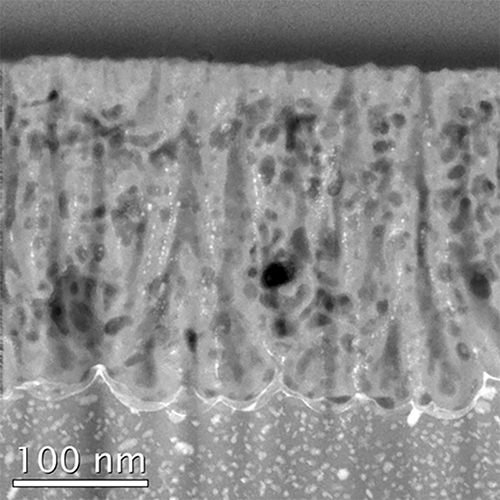
HAADF image of the cross-section of the anodized aluminum specimen prepared by anodization in sulfuric acid alone for 60 s.
