Metallurgical Abstracts on Light Metals and Alloys vol.54
Surface Modification Originated Multifunctionalization of Aluminum Alloys Using Steam
Ai Serizawa*, Yusuke Ito** and Kensuke Kurihara**
*Department of Materials Science and Engineering, Shibaura Institute of Technology
**Graduate School of Engineering and Science, Shibaura Institute of Technology
[Published in Material Science and Technology of Japan, Vol. 58 (2021), pp. 139–143]
E-mail: Serizawa[at]shibaura-it.ac.jp
Key Words:Al alloy, steam coating process, environment-friendly, corrosion resistance, strength
Al alloys, especially Al-Mg-Si alloys and Al-Zn-Mg alloys, have been used as advanced structural materials in automobile industries because of the excellent physical and mechanical properties such as low density, high specific strength and ductility. Their low corrosion resistance, however, hinders their use in the corrosive environment. To improve the corrosion resistance of the Al alloys, the development of a novel coating technology has been highly desirable. Steam coating is a novel environmentally-friendly coating method, where only pure water is required. Conventional coating methods for Aluminum alloys, anodization and chemical conversion treatments, could be replaced by steam coating. Steam coating was applied to a couple systems of Aluminum alloys. The anticorrosive film was generated with a chemical reaction of steam and Aluminum during steam coating. In this article, the mechanism of steam coating was interpreted from the chemical reaction of Aluminum alloys with steam as well as the induced precipitation behavior in substrate by a thermal energy of steam. The surfaces morphology of the specimens subjected to steam coating process was found to be significantly unique and changed depend on the process condition. These surfaces are expected to be utilized to enhance adhesion and jointing strength.
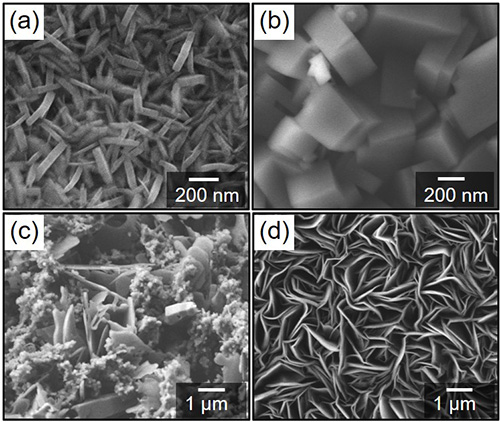
The variation of surface morphology of the specimens subjected to steam coating process. (a) Lath-like crystals, (b) block type crystals, (c) concurrent formation of lath-like and block type crystals, (d) coarse lath-like crystals.
