Metallurgical Abstracts on Light Metals and Alloys vol.54
Electroless Deposition of Aluminum from AlCl3/LiAlH4/Ether Solvents (1)~ Optimization of deposition conditions ~
Takao GUNJI*, Takeru UI*, Tatsuya WATANABE*, Fuma ANDO* and Futoshi MATSUMOTO*
*Department of Material and Life Chemistry, Faculty of Engineering, Kanagawa University
[Published in Journal of the Surface Finishing Society of Japan, Vol. 71 (2020), pp. 521–529]
https://www.jstage.jst.go.jp/article/sfj/71/8/71_521/_article/-char/en
E-mail: fmatsumoto[at]kanagawa-u.ac.jp
Key Words:Electroless deposition, Aluminum, High brightness surfaces, Additive, Cup-stacked carbon nanotube
In this study, electroless deposition of aluminum (Al) was examined with baths of AlCl3/LiAlH4/ether solvents and catalysts formed on substrate surfaces. To deposit an electroless thick Al layer, a Cu substrate, a Ti compound catalyst and diethyl ether or dibutyl ether solvents should be used for the deposition process. Al deposits with a thickness of 15 µm for a deposition time of 2 h were obtained. The rate of Al deposition was 15±2 µm h-1. The addition of dicarboxylic acids to the bath, such as glutaric acid and thiomalic acid, could improve the brightness of the deposited Al surface. As an example of an application for electroless Al, the deposition of Al on the surface of cup-stacked carbon nanotubes with diameters from 30-50 nm was successfully conducted with a bath comprising AlCl3/LiAlH4/diethyl ether and a Ti catalyst.
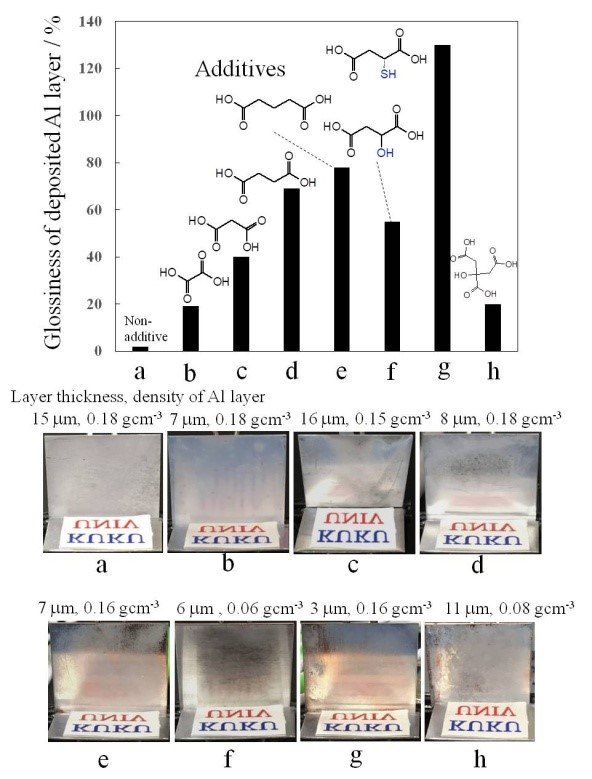
Glossiness of the Al layer surface deposited from the AlCl3/LiAlH4/dibutyl ether bath containing additives.
