Metallurgical Abstracts on Light Metals and Alloys vol.54
Initial Formation Kinetics of Calcium Phosphate on Titanium in Hanks' Solution Characterized Using XPS
Akari Hiji*, Takao Hanawa**,***, Masaya Shimabukuro****, Peng Chen**, Maki Ashida** and Kunio Ishikawa****
*Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University
**Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University
***Center for Advanced Medical Engineering Research and Development, Kobe University
***Department of Biomaterials, Graduate School of Dentistry, Kyushu University
[Published in Surface and Interface Analysis, Vol. 53 (2021), pp. 185–193]
https://doi.org/10.1002/sia.6900
E-mail: hanawa.met[at]tmd.ac.jp
Key Words: calcium phosphate, formation kinetics, hard-tissue compatibility, titanium, XPS
One cause of the excellent hard-tissue compatibility of Ti and Ti alloys compared with other metals is their ability to form calcium phosphate in biological environments. This is confirmed by many studies, although the formation mechanism has not been completely elucidated. In this study, to elucidate the initial formation kinetics of calcium phosphate on Ti in the human body, Ti was immersed in a simulated body fluid, Hanks‘ solution, for 100-106 s, followed by precise characterization using XPS. Ti specimens immersed in diluted Hanks‘ solutions were also characterized. The results reveal that phosphate ions are preferentially adsorbed, and are incorporated onto the Ti surface in 100-102 s. This reaction is slow, and the apparent thickness of the surface layer is almost constant as 5.2 nm until 102 s. However, both calcium and phosphate ions are then rapidly incorporated, and calcium phosphate is formed after 103 s. The amounts of both calcium and phosphate increase with the logarithm of time because calcium and phosphate ions react directly with the Ti surface until 105 s. Other elements contained in Hanks‘ solution are not incorporated, calcium phosphate being formed preferentially. The incorporation of calcium is faster than that of phosphate, and the [Ca]/[P] ratio increases with the logarithm of time after 103 s. However, the chemical state of surface oxide film itself on Ti does not changed by immersion in Hanks‘ solution. The formation kinetics of calcium phosphate on Ti in a simulated body fluid are clearly revealed by this study.
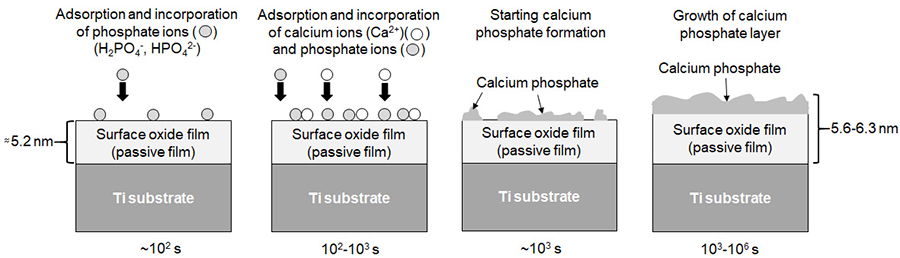
Illustration of formation process of calcium phosphate on Ti in Hanks and estimated reaction scheme. The interface between the surface oxide film and calcium phosphate is fuzzy.
