Metallurgical Abstracts on Light Metals and Alloys vol.55
Corrosion behavior of A6061 aluminum alloy in cation containing aqueous medium
Md. Saiful Islam*, ** and Masatoshi Sakairi***
*Department of Applied Chemistry & Chemical Engineering, University of Rajshahi
**Graduate School of Engineering, Hokkaido University
***Faculty of Engineering, Hokkaido University
[Published in Corrosion Communications, Vol. 5 (2022), pp. 39–48]
https://doi.org/10.1016/j.corcom.2022.01.001
E-mail: msakairi[at]eng.hokudai.ac.jp
Key Words: aluminum alloy, passive films, SEM, XPS, EIS
Though the A6061Al alloy has good corrosion-resistant, in freshwater aqueous medium chloride ions destroy the oxide film, and accelerate the corrosion reaction of Al alloys. Because freshwater contains some cations together with chloride ions. There are number of studies have been focused on the corrosion of A6061 alloy. From previous study, it can be said that the ions present in the aqueous medium are more effective in preventing corrosion than the elements contained inside the alloy. The A6061 alloy contains different elements including Mg and Zn. It is still not clear to everyone how Al alloys corrode in long-term immersion at room temperature. Moreover, if this alloy immersed in the solutions that contains Mg2+ and Zn2+ then how does it react with those ions, and what will be the oxidation condition of the alloy, these matters are unidentified. Therefore, the present study aims to clarify the corrosion natures of A6061 aluminum alloy in the stated circumstances and propose a mechanism of corrosion depending on the experimental outcomes. To accomplish the aspiration, a relative experimental condition was created, and the impact of metal cations on the corrosion of the alloy in a simulated aqueous medium was investigated with scanning electron microscope (SEM), energy dispersive X-ray spectroscope (EDS), X-ray photoelectron spectroscope (XPS), potentiodynamic polarization, and electrochemical impedance spectroscopy (EIS). The mass of the specimen was increased after different interval of the immersion tests, and a lower mass change was observed in Zn2+ containing solution in all the cases. SEM observations showed deposited product on the Al alloy surface, and comparatively, smooth surface was observed of the specimen immersed in the Zn2+ containing solution. XPS results Fig. also confirmed that Zn2+ existed on the Al alloy surface as hydroxides after immersion in the zinc ion containing solution. The Zn2+ formed a layer on the alloy surface that retarded the both the chloride attack and the corrosion reactions of the alloy.
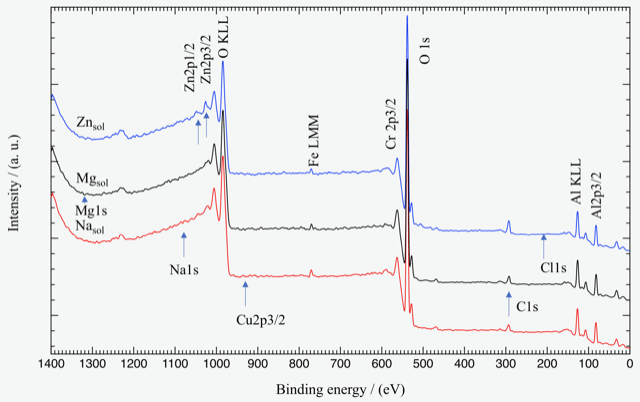
Fig. XPS wide scan spectra of specimens surface after immersed in the solutions for 7 d at 25°C.
