Metallurgical Abstracts on Light Metals and Alloys vol.55
Band Structures of Passive Films on Titanium in Simulated Bioliquids Determined by Photoelectrochemical Response: Principle Governing the Biocompatibility
Seong-Cheol Kim*, Takao Hanawa*,**,***, Tomoyo Manaka****, Hiroaki Tsuchiya*, Maki Ashida** and Shinji Fujimoto*
*Division of Materials and Manufacturing Science, Graduate School of Engineering, Osaka University
**Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University
***Center for Advanced Medical Engineering Research and Development, Kobe University
***Graduate school of Medical and Dental Science, Tokyo Medical and Dental University
[Published in Science and Technology of Advanced Materials, Vol. 23 (2022), pp. 322-331]
https://doi.org/10.1080/14686996.2022.2066960
E-mail: hanawa.met[at]tmd.ac.jp
Key Words: titanium, passive film, Hanks’ solution, saline, photocurrent, XPS, band structure, band gap, biocompatibility
The band structures and band gap energies, Eg, of passive films formed on titanium (Ti) in simulated bioliquids, Hanks’ solution (Hanks) and saline, were evaluated. Ti was polarized at 0, –0.1, and –0.2 VAg/AgCl, Ef, for 1 h. After polarization, the surfaces were characterized using X-ray photoelectron spectroscopy, and the photoelectrochemical responses were evaluated. The current change during photoirradiation was recorded as a photocurrent transient at each measuring potential, Em, and by changing the wavelength of the incident light. Passive films consisted of a very thin TiO2 layer containing small amounts of Ti2O3 and TiO, hydroxyl groups, and water. During polarization in Hanks, calcium and phosphate ions were incorporated or formed calcium phosphate but not in saline. Calcium phosphate and hydroxyl groups influenced the band structure. Eg was graded in Hanks but constant in saline, independent of Ef and Em. The passive film on Ti behaved as an n-type semiconductor containing two layers: an inner oxide layer with a large Eg and an outer hydroxide layer with a small Eg. In Hanks, Eg was 3.3–3.4 eV in the inner oxide layer and 2.9 eV in the outer hydroxide layer. In saline, Eg was 3.3 eV in the inner layer and 2.7 eV in the outer layer. Calcium phosphate and hydroxyl groups influenced the band structure of the passive film. The Eg of the outermost surface was smaller than that of TiO2 ceramics, which is probably one of the principles of the excellent biocompatibility of Ti among metals.
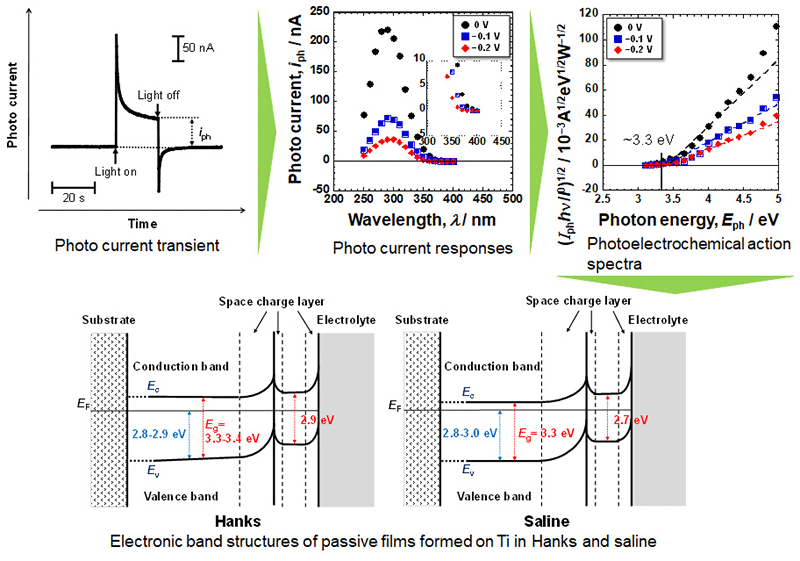
Photo current transient, photocurrent responses, calculated photoelectrochemical action spectra, and the resultant electronic band structures of passive films formed on Ti in Hanks and saline.
