Metallurgical Abstracts on Light Metals and Alloys vol.55
Forming Hard Anodic Films on Aluminum by Anodization in Oxalic Acid and Alcohol
Hidetaka Asoh and Takuma Sano
Department of Applied Chemistry, Kogakuin University
[Published in J. Electrochem. Soc., Vol. 168 (2021), 103506]
https://doi.org/10.1149/1945-7111/ac2ec1
E-mail: asoh[at]cc.kogakuin.ac.jp
Key Words: Anodization, Aluminum, Porous-type anodic film, Indentation hardness test
Porous anodic films have attracted wide attention because of their potential applications in various industrial fields. Reducing their porosity is expected to improve the hardness. This study focused on evaluating the effect of the electrolyte composition on the hardness of anodic alumina films formed on aluminum by anodization under mild conditions. In particular, the effect of adding alcohol (i.e., ethanol and ethylene glycol) to an oxalic acid-based electrolyte was considered. The voltage-time curve, coating ratio (i.e., film formation efficiency), morphology, and Martens hardness of the resulting anodic alumina films were clearly affected by the type and amount of added alcohol. Adding alcohol effectively suppressed the chemical dissolution of the anodic film during anodization without needing to lower the electrolyte temperature, which formed high-strength films with low porosity and thick pore walls. The Martens hardness of the formed films increased by 18% with the addition of 50 vol% ethanol and increased by 25% with the addition of 50 vol% ethylene glycol.
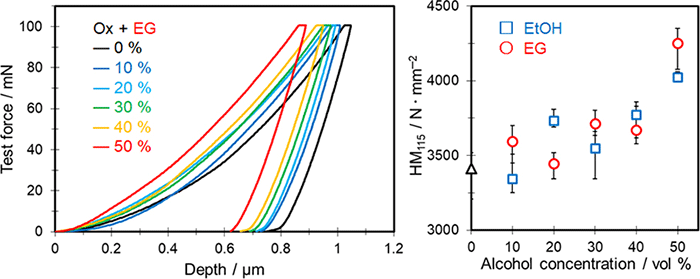
Our approach offers a new strategy for preparing hard anodic films with low porosity under mild anodization conditions to prevent burning.
