Anodic aluminum oxide with numerous nanoscale pores can be formed via anodizing aluminum in appropriate acidic or alkaline solutions and has been widely used for various applications. The nanomorphology of aluminum oxide strongly depends on the electrolyte species and operating conditions during anodizing. Recently, etidronic acid (C2H8O7P2) was investigated to possess the potential to behave as a suitable acidic electrolyte for the formation of aluminum oxide by anodizing aluminum. Although galvanostatic anodizing is typically used in industrial applications for surface finishing, very little has been reported on formation behavior during galvanostatic anodizing in etidronic acid and the nanostructure of aluminum oxide. In the present investigation, we report galvanostatic anodizing in etidronic acid under various operating parameters. Stable anodizing conditions and their corresponding voltages were examined by electrochemical measurements. In addition, nanostructural characterization of the aluminum oxide was carried out by electron microscopy and subsequent image analysis.
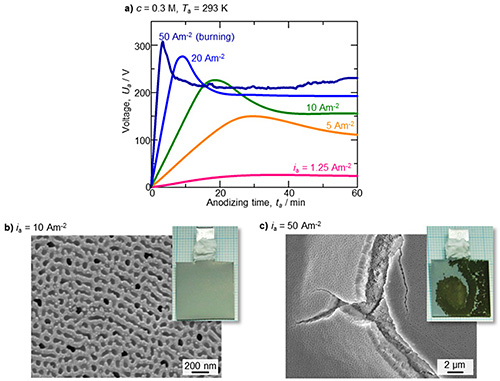
Figure 1a shows the voltage-time curves during galvanostatic anodizing in a c = 0.3 M etidronic acid solution at Ta = 298 K. At current densities of ia = 1.25-20 Am-2, typical voltage-time curves for the formation of uniform aluminum oxide were obtained. The voltage linearly increased with the anodizing time in the initial stage, reached a maximum value, gradually decreased, and then reached a plateau value. Figure 1b shows the surface appearance and corresponding SEM image of the specimen anodized at 10 Am-2 for 60 min, and a uniform, light-gray aluminum oxide film with disordered nanoscale pores measuring approximately 100 nm in diameter was obtained over the entire aluminum surface. The plateau voltage corresponding to the steady growth of the aluminum oxide increased with the current density. However, the excess current density at 50 Am-2 led to oxide burning with an unstable oscillation in the voltage-time curve, and a nonuniform dark brown oxide film with many cracks was formed on the surface (Fig. 1c).
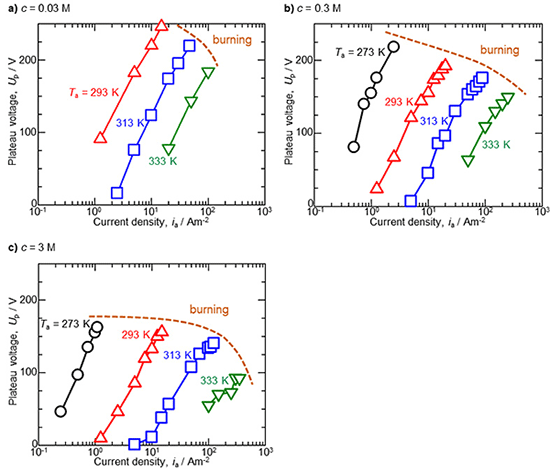
The effect of various anodizing parameters on the steady state plateau voltage during galvanostatic anodizing in etidronic acid was investigated. Figure 2a summarizes the relationship between the plateau voltage, Up, and the current density, ia, during anodizing in a 0.03 M etidronic acid solution. At a lower solution temperature of 293 K, the plateau voltage increased with the current density, and the highest plateau voltage measuring 246 V was achieved. On the other hand, the current density at this highest voltage was extremely low at 15 Am-2, and applying a higher current density of more than 20 Am-2 caused oxide burning with unstable voltage oscillation. As the temperature increased to 313 and 333 K, the plateau voltage significantly decreased to approximately more than 100 V at the same current density due to thinning of the barrier layer during anodizing at high temperature. Similar tendencies were obtained as the concentration increased to 0.3 M (Fig. 2b) and 3 M (Fig. 2c). In each concentration, the shape of the plateau voltage vs. current density was nearly linear with a slight s-shape.
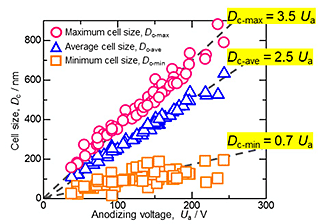
When the aluminum oxide film was dissolved from the aluminum surface in a CrO3/H3PO4 solution, the growth interface between the aluminum oxide and the aluminum substrate was exposed to the surface as an aluminum dimple array. The cell size was calculated from the SEM images of the disordered dimple array using image analysis software. Figure 3 shows the relationship between the anodizing voltage, Ua, and the calculated maximum (Dc-max), average (Dc-ave), and minimum (Dc-min) cell size of the aluminum oxide formed by galvanostatic anodizing in 0.03-3 M etidronic acid solutions at various temperatures and current densities. The dashed line shows the linear relation obtained by the least-squares method. It is clear that the average cell size linearly increased with the anodizing voltage at all concentrations. There were similar linear relationships with no dependence on the concentration and temperature. Namely, the average cell size depends only on the anodizing voltage and is directly proportional to the anodizing voltage with a proportionality constant of 2.5. Similar to the average cell size, the maximum and minimum cell sizes were directly proportional to the anodizing voltage with proportionality constants of 3.5 and 0.7, respectively.