In the present study, a new method for hydrogen charging in aluminum was developed which named friction in water (FW) process. The hydrogen charging was carried out by polishing aluminum surfaces for 1 h with a silicon carbide emery paper in water at room temperature. For comparison, hydrogen-uncharged specimens were also prepared. The total amount of hydrogen in aluminum clearly increased after the FW process, during which hydrogen was generated by the chemical reaction between water and the non-oxide coated aluminum. After hydrogen charging by the FW process, aluminum showed a decrease in ductility in conventional strain rate tensile testing. Hydrogen microprint technique (HMT) revealed that hydrogen atoms absorbed by the FW process was diffused to the opposite surface in the thickness direction.
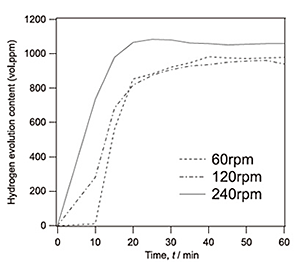
Figure 1 shows the change of hydrogen gas generation during the FW process in 99 % purity aluminum tested at rotation speeds, 30 rpm-240 rpm. Hydrogen gas generated immediately after when the stir bar with aluminum plates started to rotate, regardless of the rotation speed of stir bar. The magnitude of the hydrogen gas generation reached 900 vol. ppm after 30 min or later. In the FW process, pH value slightly changed from 6.0 to 7.5. The slight increase of pH value would be related to the formation of Al(OH)3. However, the range of pH value during the FW process was still under neutrality, thus it is evident that hydrogen generation by the FW process is caused by the chemical reaction between aluminum without oxide film and water.
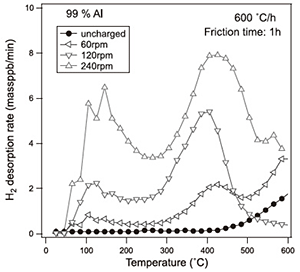
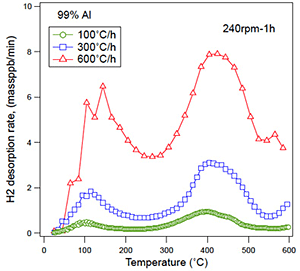
Figure 2 shows the thermal desorption analysis (TDA) of hydrogen in the specimen after the FW process for 1 h. When compared to the control specimen, the amount of hydrogen evolution was clearly increased by the FW process depending on the rotation speed of the stir bar. Typically, two peaks of hydrogen evolution were seen in the specimens with the FW process at around 150 °C and 400 °C. Figure 3 shows the effect of heating rate on the TDA curves in the specimen with the FW process by 240 rpm for 1h. The peak temperature of hydrogen evolution was shifted to higher temperatures when the heating rate was increased. The energy for hydrogen desorption from the trapping site was determined using the following equation;
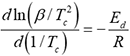
Here, β is the heating rate (K/s), Tc is the peak temperature (K), R is the gas constant (8.31 kJ/mol⋅K), Ed is the hydrogen desorption energy (J/mol). The hydrogen desorption energy corresponding to the low temperature peak (150 °C) and the high temperature peak (400 °C) were calculated to be 28.4 kJ/mol and 54.1 kJ/mol, respectively. The hydrogen desorption energy at the high temperature (54.1 kJ/mol) was almost the same value of the dislocation trapping effect for hydrogen that had been reported.
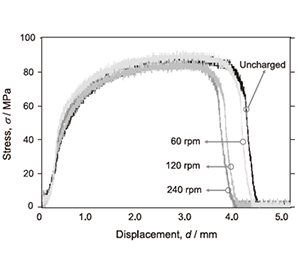
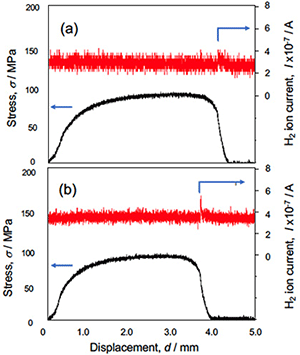
Figure 4 shows the stress-displacement curves in the specimens without and with the FW process. Total elongation was slightly decreased in the specimen after the FW process. Figure 5 shows the hydrogen gas evolution during the tensile test of aluminum without and with the FW process by 240 rpm for 1h. In the specimen with the FW process, the amount of hydrogen evolution at the moment of fracture was higher than that without the FW process. Thus, the decrease of total elongation shown in Fig. 4 would be due to the effect of hydrogen atoms which were introduced by the sequence of the FW process.