Ti and its alloys are used as implant materials in orthopedic and dental fields. Recently, an improvement in bone compatibility by forming a TiO2 layer on the Ti surface through UV irradiation has been reported,1) which is considered to be due to the cleaning of surface by decomposition of hydrocarbon contaminants through the photocatalytic activity of the TiO2 layer.1) In addition, the organic decomposition ability is related to antibacterial property. Thus, photocatalytically active TiO2 coatings help improve the bone-forming ability as well as antibacterial activity of Ti implants. It is known that anatase exhibits excellent photocatalytic activity as compared to rutile.2) Therefore, we developed a two-step thermal oxidation process for the formation of anatase-rich TiO2 on Ti substrates.3-6) In this process, the Ti substrates are treated first in a CO-containing atmosphere (first step) and subsequently in air (second step) and an anatase-rich TiO2 layer is formed on commercially pure (CP) Ti and Ti alloys. The anatase-rich TiO2 layer exhibited photocatalytic activity.3,6) In a previous study, a TiO2 layer formed on the surface of a Ti-25mass%Nb alloy (Ti-14.6 at%Nb) exhibited excellent photocatalytic activity.3) In this study, TiO2 layers were formed on Ti-xNb alloys (x = 0, 1, 10, 15, and 30 at%) by a two-step thermal oxidation process, and the photocatalytic activity of TiO2 layer under UV irradiation was investigated by measuring the water contact angle and decomposition of methylene blue (MB).
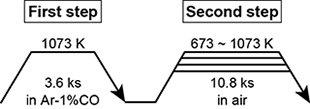
Coin-shaped Ti-xNb alloys (φ12 × 1 mm) were used for the substrates. The Nb content of the alloys and the alloy designations are summarized in Table 1. The coin-shaped Ti-xNb alloy substrates were subjected to two-step thermal oxidation, which is schematically shown in Fig. 1. The phase of the reaction layers formed on the Ti-xNb alloy surface was analyzed by α-2θ XRD and Raman spectroscopy. The reaction layer was analyzed by radio-frequency glow discharge optical emission spectroscopy (GD-OES) and XPS. A UV lamp with a peak wavelength of 351 nm was used for UV irradiation. The irradiance of the UV light source was set as 1.0 mW⋅cm-2 at the surface of the specimen. The organic decomposition ability of the reaction layer on the Ti-xNb alloys under UV irradiation was evaluated by the decomposition test of MB based on JIS R1703-2: 2007. Before UV irradiation, the specimen was placed in a 0.02 mM MB solution and stored under dark for 86.4 ks to reach an absorption equilibrium for MB on the reaction layer. After this treatment, the specimen was immersed in 2.5 mL of 0.01 mM MB solution and irradiated with UV light. The change in MB concentration on the TiO2 layer under UV irradiation was evaluated using a UV-vis spectrophotometer at 663 nm.
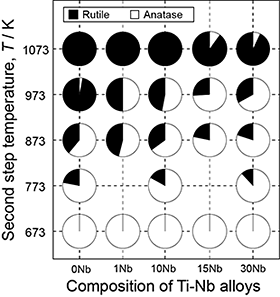
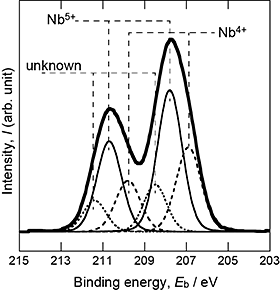
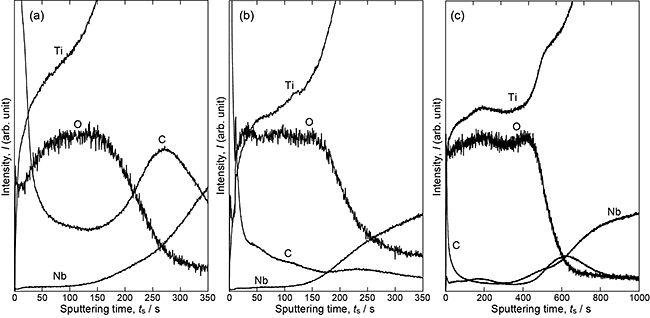
From the XRD analysis of the reaction layers, the presence of Ti(C,O) was confirmed after the first-step treatment, and the TiO2 phase was found to be the main phase of the reaction layer after the second-step treatment. Figure 2 shows the phase fraction of TiO2 as a function of Nb content in the alloys and the second-step temperature. The anatase fraction increased with decreasing second-step temperature and increasing Nb content of the alloys. Hanaor and Sorrell7) showed that the elements having large ionic radii and valence in TiO2 inhibit the transformation from anatase to rutile. Nb is classified as an inhibitor of transformation. The addition of Nb to the alloy, that is, the introduction of Nb to the TiO2 layer increased the anatase fraction under the same second-step temperature. Figure 3 shows the XPS spectrum of the TiO2 layer formed on 10Nb at the second-step temperature of 873 K. The presence of Nb5+ and Nb4+ ions in the TiO2 layer was confirmed. Nb would be dissolved into TiO2 because of the inhibition of the anatase-to-rutile transformation and the appearance of Nb signals in the XPS spectrum. The depth profiles of oxygen, carbon, and Nb of the reaction layers of 10Nb after the second-step treatment at 673, 873, and 1073 K are shown in Fig. 4. Compared with the intensity of the TiO2 region in the GD-OES profile, it is suggested that the carbon content decreased and the Nb content increased with increasing second-step temperature. In our previous study,3) both carbon dissolution and the existence of amorphous carbon/disordered graphite in the anatase layer were suggested after two-step thermal oxidation.
The rate constant of MB decomposition (k) was calculated using the concentration of the MB concentration of initial (C0) and that after UV irradiation (C) for t s in eq. (1).
ln(C/C0) = –k ⋅ t
(1)
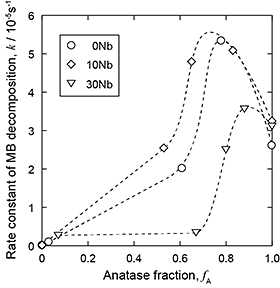
Figure 5 shows the k values as a function of anatase fraction in TiO2. The k value shows a maximum in the range of fA = 0.6 - 0.8, independent of the Nb content of the alloys. Bickley et al.8) proposed a photocatalytic mechanism of Degussa P25, which has an anatase fraction of 0.8, wherein the electron-hole separation between rutile and anatase resulted in the suppression of the recombination of the excited electrons and holes. It is suggested that this mechanism is applicable to the anatase-rich TiO2 layer prepared by the two-step thermal oxidation of Ti-xNb alloys. A comparison of the k values of 0Nb and 10Nb with an anatase fraction of around 0.5 reveals that 10Nb exhibits higher k values than 0Nb does. The Nb5+ ions introduced into TiO2 could trap the excited electrons (eq. (2)), thereby suppressing the recombination of the excited electrons and holes.9)
Nb5+ + e– = Nb4+
(2)
On the other hand, the k values of 30Nb are less than those of 10Nb and 0Nb. The effect of Nb addition to TiO2 on its photocatalytic activity has been studied.10) It was reported that Nb addition improves the photocatalytic activity of TiO2 up to a certain concentration; however, further addition of Nb deteriorates the photocatalytic activity, which agrees with the present results. Our study suggests that the TiNb2O7 formed in TiO2 possibly acts as a site of recombination of the excited electrons and holes in the case of 30Nb.
1) H. Aita et al.: Biomaterials 30 (2009) 1015–1025.
2) P. Frach et al.: Vacuum 80 (2006) 679–683.
3) S. Sado et al.: Appl. Surf. Sci. 357 (2015) 2198–2205.
4) T. Okazumi et al.: J. Mater. Sci. 46 (2011) 2998–3005.
5) N. Umetsu et al.: Mater. Trans. 54 (2013) 1302–1307.
6) T. Ueda et al.: Mater. Lett. 185 (2016) 290–294.
7) D.A.H. Hanaor and C.C. Sorrell: J. Mater. Sci. 46 (2011) 855–874.
8) R.I. Bickley et al.: J. Solid State Chem. 92 (1991) 178–190.
9) S.E. Park et al.: Sol. Energy Mater. Sol. Cells 83 (2004) 39–53.
10) B.K. Kaleji et al.: Mater. Chem. Phys. 132 (2012) 210–215.