Hydrogen embrittlement sensitivity (HES) of Al-Zn-Mg base alloys with high amount of zinc was studied by preparing the alloys without and with hydrogen charging. In order to clarify the effect of source of hydrogen entry on HES, we compared tensile properties between conventional strain rate testing (CSRT) in an ultrahigh vacuum atmosphere, and slow strain rate testing (SSRT) in a humid air atmosphere. It was clarified that the HES of the Al-Zn-Mg alloys varied depending on the source of hydrogen entry. Namely, internal hydrogen introduced during the melting and cast process lowers strength of the alloys. On the other hand, external hydrogen introduced from the alloy surface during SSRT under humid air raised the HES in the alloy with high strength. The HES caused by internal hydrogen was increased when the Al-Zn-Mg alloy contained high amount of hydrogen plus 0.1% of iron. The HES caused by external hydrogen was increased when the peak-aged Al-Zn-Mg alloy contained 0.1% of iron.
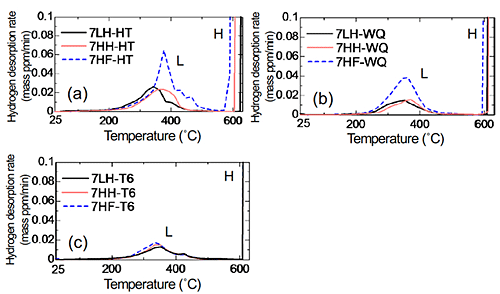
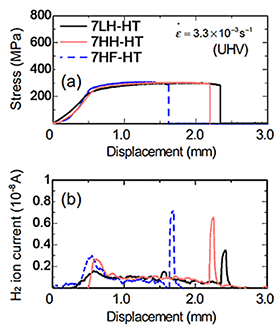
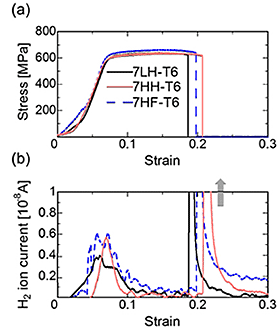
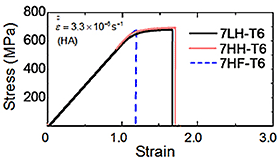
Figure 1 (a) shows the TDA curves of Al-Zn-Mg-Cu alloys. (a): HT, (b): WQ, (c): T6. As a whole, hydrogen evolution peaks were observed around 250 °C-450 °C (peak L) and above 580 °C (peak H). In comparison with the previous reports, it is believed that the peak L is from the hydrogen trapping by dislocations and grain boundaries, and that the peak H is from that by micropores or voids. When the heat treatment state is changed from homogenization to peak-aging, the hydrogen evolution decreased. However, even after peak-aging condition, the order of the hydrogen content was maintained. The alloy with high amount of iron evolved higher amount of hydrogen after homogenization and solution heat treatment. Figure 2 shows CSRT and hydrogen evolution behavior in an UHV atmosphere of Al-Zn-Mg-Cu alloys (HT). In the HT, decrease of ductility was observed when the internal hydrogen content of the alloy was high, in spite of that the proof stress and tensile strength were unchanged. Hydrogen gas evolution during the tensile test was observed in the beginning of plastic deformation and at the moment of fracture. The hydrogen gas evolution in the plastic deformation would be related to the hydrogen transformation by mobile dislocations, and that in the fracture was caused by hydrogen evolution from fracture surface. Figure 3 shows CSRT and hydrogen evolution behavior in an UHV atmosphere of Al-Zn-Mg-Cu alloys (T6). In the T6, the decrease of mechanical properties was not observed regardless of the difference of internal hydrogen content and additional iron. The hydrogen evolution in the T6 specimens was much higher than that in the HT condition. This suggests that hydrogen evolution was enhanced by the increase of stress level during deformation. In the T6 condition, fracture surfaces after the test were fully covered with grain boundary fracture. From this, detrimental effect of hydrogen on mechanical property is relatively low because of the higher effect of aging precipitates on grain boundary fracture. Figure 4 shows the SSRT in a humid air (HA) atmosphere of Al-Zn-Mg-Cu alloys (HT). Similary to the case of CSRT, the alloys with higher hydrogen content showed the decrease of ductility. In this case, it is assumed that environmental hydrogen introduced from air atmosphere plus internal hydrogen both affected the decrease of ductility. Figure 5 shows the SSRT in a humid air (HA) atmosphere of Al-Zn-Mg-Cu alloys (T6). Contrary to the case of CSRT, the alloys with higher hydrogen content and iron showed the decrease of ductility. The ductility between 7LH and 7HH was almost the same. This suggest that hydrogen introduced from air atmosphere strongly affected the decrease of ductility, rather than internal hydrogen.