This work reports for the first time the effect of femtosecond laser shock peening (fLSP) without a sacrificial overlay under atmospheric conditions (no confinement) on the corrosion behaviour of AA2024-T3. After fLSP and additional exposure to air a superhydrophobic state (θ=160±4°) is achieved. Moreover, fLSPed corrosionresistant surface contributes to increased polarization resistance, reduced corrosion current and lower anodic dissolution, with long-term stability in aggressive chloride solution. Furthermore, SEM/EDS characterization reveals reduction in pitting and complete eradication of intergranular corrosion (IGC) attack due to reduced metal/electrolyte contact area and homogeneous, refined microstructure which prevents chain-link IGC propagation.
Figure 1 illustrates schematically the experimental setup for femtosecond laser peening. The specimen of AA2024-T3 was mounted on an x–y stage as shown in Fig. 1a. Femtosecond laser pulses (Spectra-Physics Inc., Spitfire) with a wavelength of 800 nm, a pulse width of 120 fs and pulse energy of 0.6 mJ were focused using a plano-convex lens with a focal length of 70mm and irradiated normal to the electropolished surface of the specimen in air. For the peening treatment, the aluminium specimen was moved in the x- and y-directions during laser irradiation as shown in Fig. 1b to be irradiated with a coverage Cv of 692% which is expressed by Cv=![]() D Np/4 2 where D is the spot diameter of the laser pulse irradiated and Np is the number of pulses per unit square.
D Np/4 2 where D is the spot diameter of the laser pulse irradiated and Np is the number of pulses per unit square.
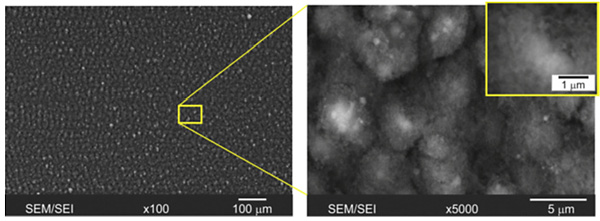
Figure 2 shows the SEM images of the femtosecond laser-peened surface of the AA2024-T3 with multidirectional structures as a consequence of constant melting, ablation and deposition of the recast material during laser treatment. Nonetheless, droplets were not observed, indicating that the femtosecond laser treatment creates a negligibly small molten layer. The surface roughness Ra is 1.2 µm, which is more than 2-times lower than that of a nanosecond laser-peened as well as shot-peened surface, respectively.
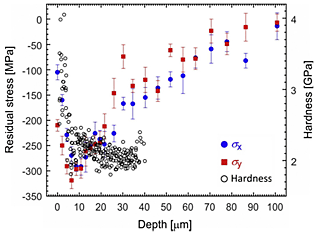
Figure 3 depicts the residual stress along the x-direction σx and y-direction σy, and hardness as a function of depth from the surface. The surface region within around 100 µm has a compressive residual stress for both x- and y-directions, of which maximum value is about 300 MPa at a depth of 6 µm, which is almost same as the 0.2% proof stress of AA2024-T3. The region within 6 µm from the surface with the most compressive residual stress corresponds to the most hardened region. The largest value of the hardness is almost twice that of the unpeened aluminium alloy with a hardness of 2.0 GPa, which indicates that local work-hardening or plastic deformation induces the compressive residual stress.
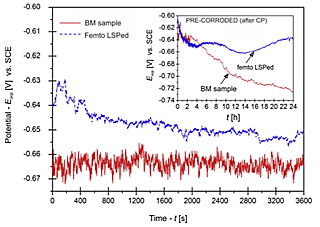
The OCP curves (Eocp=f[t]) of the femto laser-peened (fLSPed) and BM samples in a 0.6M NaCl aqueous solution are depicted in Fig. 4. Potential-time transients indicate more anodic (positive) values of the fLSPed sample compared to the BM sample, with relatively stable potential values with lower fluctuations after approximately 30 min immersion in the test solution. The initial OCP values were –640 mVSCE and –665 mVSCE for fLSP and BM samples, respectively. Afterwards, a small gradual potential decrease with longer immersion time was observed, which after 1 h immersion achieved a potential of –650 mVSCE and –663 mVSCE.
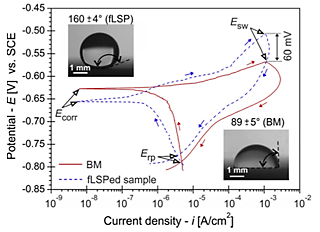
Electrochemical characteristics are analysed via CP curves to study active/passive behaviour and the propensity of material towards pitting corrosion. CP curves of non-processed (BM) and fLSP AA2024-T3 samples with the insert of water droplets obtained during the wettability measurements (prior to corrosion experiments) are shown in Fig. 5.
Femtosecond laser shock peening (fLSP) without a sacrificial overlay under atmospheric conditions with no confining medium was confirmed as an effective method to induce high compressive residual stress and increased hardness in the treated, top-surface layer. Moreover, a hierarchical superhydrophobic surface (SCA=160±4°) with almost negligible roughness increased and improved corrosion behaviour in a 0.6M NaCl test solution.
OCP results during 1 h immersion confirmed potential ennoblement of the fLSPed sample, whereas during longer 24 h immersion time on pre-corroded samples after CP, reduced corrosion activity was also confirmed, with improved long-term stability of fLSPed surface, due to more resistant oxide film. LPR and CP results confirmed 18-times higher polarization resistance Rp (34.83 kΩcm2 vs. 1.92 kΩcm2) and 4-times lower corrosion current density icorr (1.95 µm-2 vs. 0.49 µAcm-2) for the fLSPed sample. Moreover, a lower degree of anodic dissolution, improved and faster repassivation, with more than 150% higher ΔE/Δi gradient was confirmed for the fLSPed sample, indicating much lower anodic dissolution in aggressive Cl- solution.
SEM/EDS analyses of the BM sample revealed extensive, widespread localised pitting and IGC attack. In contrast, for the fLSPed sample the pitting attack was confined to a few isolated regions, whereas the initiation of IGC was completely eradicated due to reduced fractional area of liquid–solid interface and a more homogeneous, refined microstructure with evenly distributed constituent particles, which prevented the ‘chain-link’ IGC effect. In addition, EIS results demonstrated good corrosion inhibitive properties of the fLSPed sample, similar to the LPR and CP results, with higher total resistance. Moreover, EIS results have shown similar behaviour of ‘fresh’ and pre-corroded fLSPed samples, with smaller variations of circuit parameters than the BM sample, indicating a durable surface with improved long-term stability against corrosion in a chloride environment.