Al alloys, especially Al-Mg-Si alloys and Al-Zn-Mg alloys, have been used as advanced structural materials in automobile industries because of the excellent physical and mechanical properties such as low density, high specific strength and ductility. Their low corrosion resistance, however, hinders their use in the corrosive environment. To improve the corrosion resistance of the Al alloys, the development of a novel coating technology has been highly desirable. Steam coating is a novel environmentally-friendly coating method, where only pure water is required. Conventional coating methods for Aluminum alloys, anodization and chemical conversion treatments, could be replaced by steam coating. Steam coating was applied to a couple systems of Aluminum alloys. The anticorrosive film was generated with a chemical reaction of steam and Aluminum during steam coating. In this article, the mechanism of steam coating was interpreted from the chemical reaction of Aluminum alloys with steam as well as the induced precipitation behavior in substrate by a thermal energy of steam.
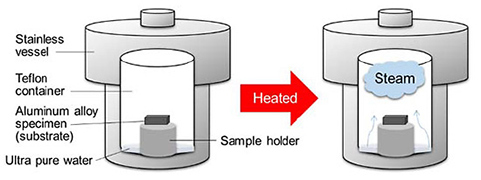
An Al-Mg-Si alloy (A6022) and an Al-Zn-Mg alloy (A7075) with a size of 20 mm ![]() 20 mm were used as the substrate. The steam coating was performed using a horizontal autoclave made of stainless steel with a volume of 10 m3. The autoclave was a cylindrical with a diameter of 1.5 m and the length of 2.7 m. Ion-exchanged water was used as the steam source. A flow of temperature-controlled steam between 160 °C and 180 °C was introduced into the autoclave. The pressure was also set to 0.6 MPa at 160 °C, 0.75 MPa at 170 °C, and 0.95 MPa at 180 °C. The steam coating process was conducted for 0.5–1 h. Once the required holding time had elapsed, the autoclave was allowed to cool naturally to room temperature, resulting in the formation of an anticorrosive film on the Al alloys. A schematic illustration of the steam coating technology is shown in Fig. 1.
20 mm were used as the substrate. The steam coating was performed using a horizontal autoclave made of stainless steel with a volume of 10 m3. The autoclave was a cylindrical with a diameter of 1.5 m and the length of 2.7 m. Ion-exchanged water was used as the steam source. A flow of temperature-controlled steam between 160 °C and 180 °C was introduced into the autoclave. The pressure was also set to 0.6 MPa at 160 °C, 0.75 MPa at 170 °C, and 0.95 MPa at 180 °C. The steam coating process was conducted for 0.5–1 h. Once the required holding time had elapsed, the autoclave was allowed to cool naturally to room temperature, resulting in the formation of an anticorrosive film on the Al alloys. A schematic illustration of the steam coating technology is shown in Fig. 1.
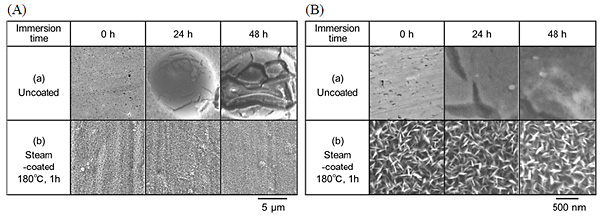
SEM images showing the surfaces of the specimens subjected to steam coating at 160 °C, 180 °C, and 200 °C, for various durations, are shown in Fig. 2. Needle or lath-like crystals were clearly observed on the surfaces of all the specimens. The crystals completely covered the surface, and the distribution of the crystal size was small, regardless of the process conditions. The typical length of the crystals was 50–300 nm, while their width was 14–20 nm. Both the length and width increased slightly with the treatment temperature and holding time.
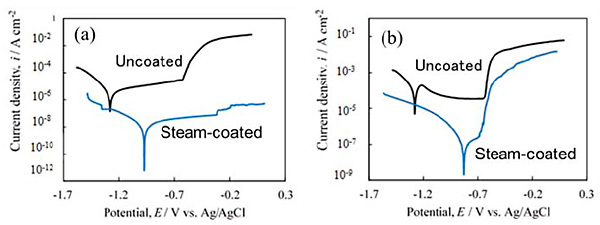
The corrosion resistance of the steam-coated film on the Al-Mg-Si alloy and the Al-Zn-Mg alloys were evaluated by electrochemical measurements. The following is an example of the evaluation of the corrosion resistance of the film-coated Al alloys, as determined by electrochemical measurements. The corrosion resistance of the film formed on Al-Mg-Si was determined from potentiodynamic polarization curve measurements. The potentiodynamic polarization curves of those specimens subjected to steam coating at 180 °C for 6 h are shown in Fig. 3. As a control, the potentiodynamic polarization curve of untreated Al-Mg-Si alloy or Al-Zn-Mg alloy are also shown in Fig. 3. An immersion corrosion test was performed to evaluate the pitting corrosion properties of the bare Al-Mg-Si alloy and the film-coated Al-Mg-Si alloy. Low-magnification and high-magnification SEM images showing the surface morphologies of (a) the uncoated specimen and (b) the specimen subjected to steam coating at 180 °C for 1 h, after immersion in salt water for various durations, are shown in Figs. 4 (A) and 4 (B), respectively. The appearance of the surface of the specimen that was subjected to steam coating at 180 °C for 1 h exhibited no damage at all, even after saltwater immersion for 48 h. In contrast, considerable pitting corrosion was observed on the as-received specimen, even after only 6 h of saltwater immersion.
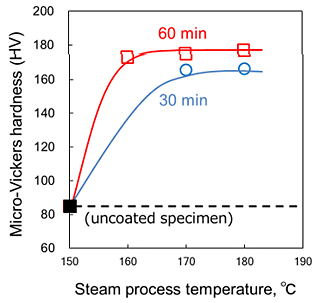
The hardness of the uncoated and f-coated Al-Zn-Mg alloys were evaluated by Micro-Vickers hardness tests. The change in Micro-Vickers hardness of uncoated specimen and specimen conducted to steam coating 180 °C for 0.5–1 h are shown in Fig. 5. The microhardness of the steam-coated specimen showed more than 180 HV, suggesting that the thermal energy of steam during steam coating process induced the precipitation hardening of the substrate of Al alloy.