Multilateralization, i.e. usage of various kinds of metallic structural materials for the appropriate parts, is one of effective techniques to reduce the exhaust amount of CO2 to operate airplanes, trains and automobiles. The importance of dissimilar metal joining has been increasing as never before, because joining of dissimilar materials is indispensable for multimaterialization. However, demonstration of dissimilar metal joining widely applicable to various metals is often difficult except for some specialized condition, even though typical combination of metals, e.g. steel and aluminum alloys.
Dissimilar metal joining is difficult because of the following two typical reasons. First, thermal stress, which caused during cooling by difference between welding temperature and ambient temperature, break nuggets. Second, brittle intermetallic compounds are formed in the nugget of dissimilar metal joining. The solution to solve the above two reasons is to use inserted materials between the dissimilar metals.
Various combinations of dissimilar metals and inserted materials have been proposed. But, new inserted materials should be explored whenever new combinations of dissimilar metals are tried to be joined. To promote dissimilar metal joining for producing industrial goods, a new inserted material that is available for various pair of dissimilar metals should be developed.
Therefore, inserted materials that increase supercooling ability of nuggets can be applicable to various types of dissimilar metal welding, because rapidly cooling is accompanied by welding. If the supercooling ability of the nugget is increased, solidification (i.e. crystallization) temperature decreases. It results in decreasing of the temperature difference causing thermal stress. The typical candidates for inserted materials with high supercooling ability are the Zr-Cu-Al ternary alloys. The Zr-Cu-Al ternary alloys exhibit low eutectic temperature. Thus, the solidification temperature is drastically decreased when the composition of the nugget is close to the eutectic point.
Moreover, non-equilibrium phases, such as a metallic glass phase with ultra-high strength or high entropy alloy phase with high ductility, may be formed in a nugget if the nugget has high supercooling ability, in place of brittle intermetallic compounds. Those alloys with high supercooling ability can exchange atoms by diffusion with the metals to be welded, and resulting in high joining strength, because they are often composed of more than three elements, some of them are similar to the element of the metals to be welded.
Among various joining techniques, resistance spot welding is widely applicable to various purposes. So, resistance spot welding becomes an indispensable technique to dissimilar joining, if the nugget with high supercooling ability can suppress thermal stress and intermetallic compounds. In this study, the stacking of Zr, Cu and Al foils were used as inserted materials for dissimilar resistance spot welding of SUS304 and CP-Ti plates in order to increase supercooling ability of nuggets.
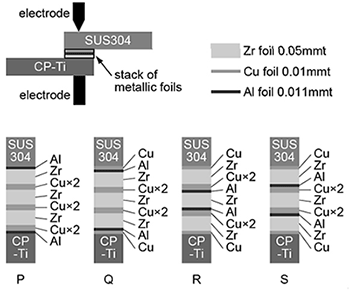
SUS304 and CP-Ti plates were subjected to resistance spot welding with using stacking of Zr, Cu and Al foils (Fig. 1). Five types of dissimilar resistance spot welding of SUS304 and CP-Ti plates were demonstrated with and without the stacking of Zr, Cu and Al foils. The thickness of the Zr, Cu and Al foils were 0.05 mm, 0.01 mm and 0.011 mm, respectively. The numbers of the foils were chosen so that the composition of the stacking of the foils becomes Zr50.2Cu39.5Al10.3, which is almost the same composition of the Zr50Cu40Al10 metallic glass with high supercooling ability. Four types of the stacking sequence of the foils were examined for the resistance spot welding (specimen P, Q, R and S). Mechanical properties of the four types of the specimens of the foils were examined by tensile shear testing. Five test pieces were subjected to tensile shear testing for each type of the stacking sequence.
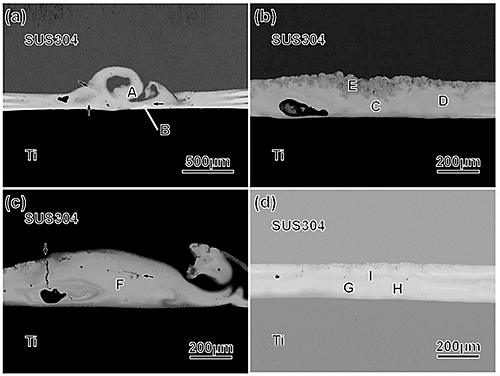
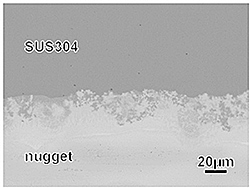
No cracks and few cavities were found in the nugget when the Zr, Cu and Al foils were stacked in one of the four sequences, specimen S, (Fig. 2(d)). The composition of the nugget was close to Zr50Cu40Al10, thus it is expected that the nugget contains a metallic glass phase, which was formed during welding by rapidly quenching. On the contrary, the nuggets of the three other specimens, P, Q and R, contained some cracks and cavities (Figs. 2 (a-c)). The maximum strength for the specimen whose nugget had no cracks and few cavities ranged 205 to 276 MPa. The difference in the maximum strength of this specimen, S, was smaller than that of the three other specimens, P, Q and R. It is considered that the metallic glass phase formed in the nugget unified the condition during welding for each specimen. The boundary between the nugget and the SUS304 plate was wavy by diffusion, while that between the nugget and the CP-Ti (Fig. 3). To increase the maximum strength, diffusion at the boundary between the nugget and the CP-Ti should be promoted.