Ti and its alloys have several applications, such as artificial joints, bone plates, and dental implants, as biomedical materials because of their excellent corrosion resistance, mechanical properties, and biocompatibility. Ti-6Al-4V is the most commercially available α+β-type Ti alloy for use in not only biomedical devices but also many fields, such as aerospace components and chemical plants. It is well known that the production cost of Ti and its alloys is high, and the utilization of ubiquitous elements has been suggested as an effective method of cost reduction for Ti alloys. Oxygen, a ubiquitous element and the main impurity in a Ti sponge. Therefore, utilizing oxygen as an alloying element lowers the production cost of Ti alloys as low-grade Ti sponge and Ti scrap can be employed as raw materials. In this study, we focused on Nb as an alloying element of low-cost biomedical α+β-type Ti alloy. Nb is a β stabilizer and β-isomorphous element with high oxygen solubility. It is also used as an alloying element for biomedical β type Ti alloys. In this study, to develop biomedical α+β-type Ti-Nb-O alloys, the effect of Nb and oxygen on the microstructure and mechanical properties was investigated.
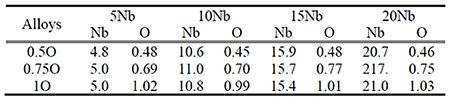
Ti-Nb-O alloys with four Nb levels and three oxygen levels were fabricated by arc melting. Chemical composition of the alloy is shown in Table 1. The ingot was forged in the α+β region at 1073 K to a φ8 mm bar, and again followed by air cooling to room temperature. The heat treatments were conducted in an Ar flow atmosphere at 873–1373 K for 3.6 ks, followed by ice-water quenching.
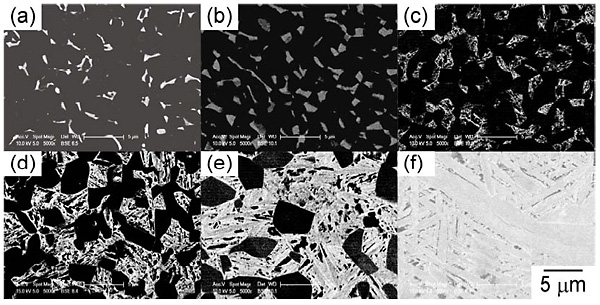
Figure 1 shows SEM microstructures of the heat-treated Ti-5Nb-0.5O alloy. The black and gray regions in the figure indicate α and β grains, respectively. Equiaxed α grains were observed in the alloys heat treated at 973 to 1173 K but not after heat treatment at 1223 K. Furthermore, the transformed microstructure, which shows acicular morphology, was observed after quenching from 1073 to 1223 K.
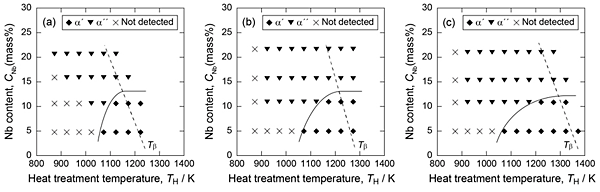
The constituent phases of the alloys were analyzed through XRD. The formation conditions of the α’ and α” martensites in the alloys are summarized in Fig. 2. In the case of the alloys with 0.5-mass% oxygen content (Fig. 2(a)), the formation of α’ martensite was observed in the 5Nb alloy. In the 10Nb alloy, both α’ and α” martensites were formed. In the 15Nb and 20Nb alloys, although the formation of α” martensite was detected, α’ martensite was not. In the case of the alloys with oxygen content of 0.75 and 1 mass%, the formation of α’ and α” martensites were observed in 10Nb alloys, and the boundary temperature for their formation increased with increasing oxygen content. This suggests that Nb distribution to β phase is enhanced by oxygen addition.
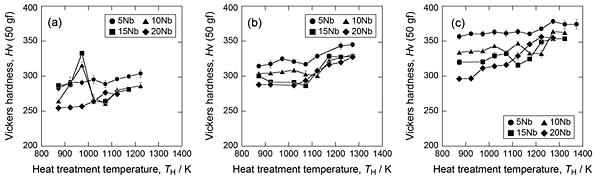
Figure 3 shows the change in Vickers hardness of the alloys with change in heat-treatment temperature. In the Ti-10Nb-0.5O and 15Nb-0.5O alloys, a significant increase in hardness was observed after heat treatment at 873–973 K and maximum hardness was obtained after heat treatment at 973 K. Under these conditions, athermal ω formed in these alloys after quenching, and its amount was larger in the alloy heat-treated at 973 K. Athermal ω is reported to increase the hardness of Ti alloy. Therefore, this study suggests that an increase in hardness of these alloys was caused by the formation of athermal ω. No significant increase in hardness was observed in the alloys with oxygen content of 0.75 and 1 mass%. These results indicate that athermal ω was not formed in the alloys with higher oxygen content because oxygen suppresses the formation of athermal ω.
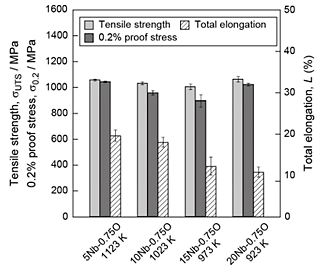
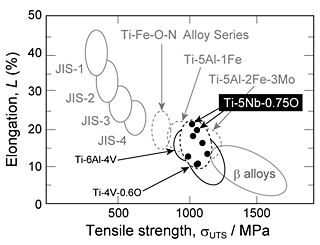
The tensile strength of the alloys with oxygen content of 0.75 mass% was tested and the fα was fixed to approximately 0.5 by changing the heat-treatment temperatures. The ultimate tensile strength (σUTS), 0.2% proof stress (σ0.2), and total elongation (L) of the Ti-xNb-0.75O alloys are shown in Fig. 4. The ultimate tensile strength of the alloys with oxygen content of 0.75 mass% was more than 1000 MPa, except for Ti-15Nb-0.75O alloy heat-treated at 973 K. The total elongation decreased with increasing Nb content. The Ti-5Nb-0.75O alloy exhibited the highest total elongation. A comparison of α+β-type Ti alloys with high oxygen content with other commercially available Ti and Ti alloys is shown in Fig. 5. The figures also show tensile properties of Ti-4V-0.6O alloy developed in our group. The comparison of the tensile properties of Ti-Nb-0.75O alloys and other Ti alloys shows that the alloy system developed in our group has comparable mechanical properties with those of Ti-6Al-4V alloys. Among Ti-xNb-0.75O alloys, the Ti-5Nb-0.75O alloy exhibits excellent strength–ductility balance as a new low-cost biomedical α+β type-Ti alloy.