Investigations of the corrosion of aluminum alloys in fresh water focused on the effects of metal cations was carried out. It was showed that the corrosion rate of A3003 aluminum alloy in fresh water is changed by the kinds of metal cations in the model fresh water where the concentration of chloride ions, dissolved oxygen, and pH are similar. The researches also elucidated that zinc ion is superior to inhibit the fresh water corrosion of A3003 alloy among the metal cations used in the studies. However, the minimum concentration of zinc ion to inhibit the corrosion of A3003 aluminum alloy in model fresh water has not been determined.
In this research, the corrosion behaviors and the corrosion products of A3003 aluminum alloy formed in fresh water with different zinc ion concentrations were investigated by immersion tests combined with surface observation and an analysis using auger electron spectroscope (AES). The high-resolution surface analysis by AES will contribute to understand the structure of corrosion products formed on A3003 after immersion tests. The objective of this study is to determine the minimum concentration of zinc ion which inhibits the corrosion of A3003 aluminum alloy in model fresh water, and to establish the details of the corrosion inhibition mechanism for the aluminum alloy in model fresh water containing zinc ion by surface observations and analysis. Corrosion mechanism and morphology changes of aluminum alloy in model solution corresponding sea water containing gluconates (organic inhibitor) and zinc ions were investigated by immersion corrosion tests with surface analysis.
The specimens were A3003-H24 aluminum alloy sheets (composition (mass%): Cu, 0.11; Si, 0.36; Fe, 0.55; Mn, 1.08; Mg, <0.01; Zn, 0.01; Ti, 0.03, Al: bal.), cut into a size of 7 ![]() 7
7 ![]() 1 mm. The specimens were embedded in epoxy resin (Struers Ltd., EpoFix Resin). The exposed surfaces of the embedded specimens were ground with silicon carbide abrasive paper from #600 to #4000 and finally polished by buffing. The following four solutions with different zinc ion concentrations, 2.0 mM NaCl (NaCl), 0.01 mM ZnCl2 + 1.98 mM NaCl (0.01Zn), 0.1 mM ZnCl2 + 1.8 mM NaCl (0.1Zn), and 1 mM ZnCl2 (1.0Zn) were used as test solutions. The concentration of chloride ion in the solutions was adjusted to 2.0 mM and the pH of the solutions were approximately 6. Specimens were immersed in the solutions for 2.59 Ms (30 d) at 298 K in bottles, with the bottles open to the air during the immersion tests. The surfaces and cross-section of the specimens were observed by an AES (JEOL Ltd., JAMP-9500 F). The specimen morphology was observed by the scanning electron microscope (SEM) function of the AES. A cross-section polisher (CP, JEOL Ltd., SM-09010) was used to prepare cross-sections of the specimens. The cross-sectional structures of the immersed specimens were also analysed by the AES. An Ar ion sputtering gun was used to clean contamination form the specimens.
1 mm. The specimens were embedded in epoxy resin (Struers Ltd., EpoFix Resin). The exposed surfaces of the embedded specimens were ground with silicon carbide abrasive paper from #600 to #4000 and finally polished by buffing. The following four solutions with different zinc ion concentrations, 2.0 mM NaCl (NaCl), 0.01 mM ZnCl2 + 1.98 mM NaCl (0.01Zn), 0.1 mM ZnCl2 + 1.8 mM NaCl (0.1Zn), and 1 mM ZnCl2 (1.0Zn) were used as test solutions. The concentration of chloride ion in the solutions was adjusted to 2.0 mM and the pH of the solutions were approximately 6. Specimens were immersed in the solutions for 2.59 Ms (30 d) at 298 K in bottles, with the bottles open to the air during the immersion tests. The surfaces and cross-section of the specimens were observed by an AES (JEOL Ltd., JAMP-9500 F). The specimen morphology was observed by the scanning electron microscope (SEM) function of the AES. A cross-section polisher (CP, JEOL Ltd., SM-09010) was used to prepare cross-sections of the specimens. The cross-sectional structures of the immersed specimens were also analysed by the AES. An Ar ion sputtering gun was used to clean contamination form the specimens.
SEM images of specimens surface after after immersion for 30 d in (a) NaCl, (b) 0.01Zn, (c) 0.1Zn, and (d) 1.0Zn are shown in Fig. 1. From the surface SEM images, significant amount of corrosion products are formed on the 1.0Zn specimen more than on the other specimens. There were no intermetallic compounds on any of the specimens like those which were observed on the specimens before the immersion tests. This means that the thick corrosion products were formed on the specimens in all the solutions and then the metallic surface of the aluminum alloy is covered by corrosion products.
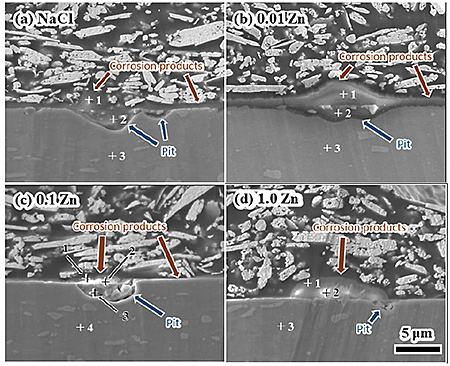
Figure 2 shows cross-sectional SEM images of specimens after immersion for 30 d in (a) NaCl, (b) 0.01Zn, (c) 0.1Zn, and (d) 1.0Zn. The cross-sectional SEM image of 1.0Zn indicates that the dome-shaped products formed in the 1.0Zn experiment also have a multi-layer structure, and that products are not formed on the pit site. These cross-sectional observations suggest that 1.0 mM Zn ions in fresh water can prevent formation of large pits in the A3003 alloys, and that the thickness of the corrosion product layer decreases with increases of zinc ion concentration in the model fresh water here.
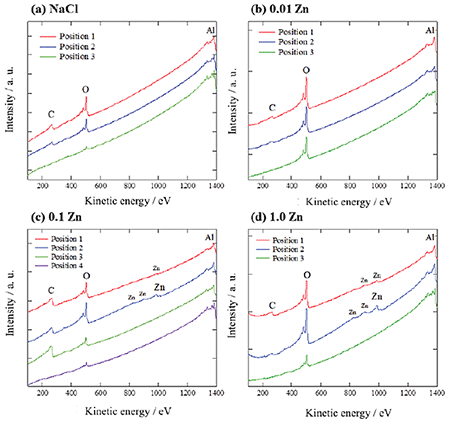
Figure 3 shows the results of the cross-sectional AES point analysis of the specimens after immersion in solutions for 30 d. Three or four different positions were analyzed on each specimen and the analytical positions are indicated (+) in Fig. 2. Zinc peaks are detected in the corrosion products formed on the pits of the specimen immersed in 0.1Zn. This indicates that the corrosion products are composed of a zinc rich layer (inner white layer) and an aluminum rich layer (outer gray layer). The results of this study indicate that the minimum concentration of Zn2+ which can inhibit corrosion of A3003 in model fresh water would be 0.1 mM.