Al-Mg alloys (5000 series Al alloys) are most widely used as practical wrought Al alloys because of their good mechanical properties at room temperature, corrosion resistance, formability, weldability, and so on. Since these alloys are solid solution strengthened alloys, the strength can be increased with increase of Mg concentration. However, there is a trade-off relationship between the strength and ductility in metallic materials, such that ductility is impaired even if the strength is increased by various methods. In the Al-Mg alloys, ductility decreases due to intergranular fracture when the Mg concentration is 5% or more. The cause of intergranular fracture is presumed to be decrease of grain boundary strength due to grain boundary segregation of Mg and to be increase of concentration of stress on grain boundary due to increase of amount of solid solution strengthening. From the above, high strength Al-Mg alloy having Mg concentration of 5% or more has not been commercialized. Al-Mg alloys with higher Mg content showing high strength and high ductility are required. In order to suppress the intergranular fracture and increase ductility, the addition of grain boundary strengthening elements is effective. It has been reported that bonding strength of grain boundary was improved by the addition of grain boundary strengthening element in steel and in Al alloys. Thus, we focused on strengthening the grain boundary by element addition as a method of suppressing intergranular fracture and increasing ductility in this research. Grain boundary strengthening elements can be predicted from first-principles calculations. Previously, grain boundary segregation energy, surface segregation energy and grain boundary strengthening / embrittling energy of solute 55 element at Σ 11 Al grain boundary have been calculated by using first-principles calculations. By using these results, we can predict the grain boundary strengthening element in Al alloys. The objective of this study is to find a grain boundary strengthening element in Al alloy, and to develop a high strength and high ductility Al-Mg alloy exceeding the trade-off balancing between strength and ductility by adding the grain boundary strengthening element to high strength Al-8Mg alloy. Since the impurity elements such as Si decrease ductility in Al alloys, ultra-high purity alloys that impurity amount has been reduced to several mass ppm or less were used in order to clarify the influence of the grain boundary strengthening element.
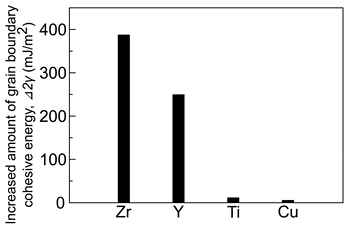
In this study, we calculated increased amount of grain boundary cohesive energy of 55 solute elements at AlΣ11(113)[110] grain boundary. When calculating increased amount of grain boundary cohesive energy by solute atoms, thermal equilibrium temperature and solute concentration in grain were set to 298 K and solubility limit of each solute element in Al, respectively. Figure 1 shows the top four elements with the largest increased amount of grain boundary cohesive energy among the 55 solute elements. It was revealed that Zr increased grain boundary cohesive energy mostly in the equilibrium state of 298 K. Based on the above results, Zr was determined as an additive element in this study.
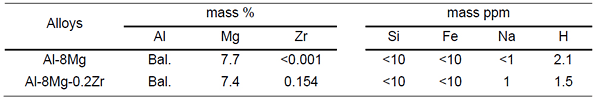
An Al-8Mg alloy made with high purity aluminum (99.999 mass% purity) and magnesium (99.99 mass% purity) and an Al-8Mg-0.2Zr alloy added 0.154 mass% Zr alloy cast material (φ30 150 mm) was used. The chemical composition of each alloy is shown in Table 1. Homogenization treatment (703 K
150 mm) was used. The chemical composition of each alloy is shown in Table 1. Homogenization treatment (703 K 16 h), surface cutting (φ30 to 22 mm), hot swage (473 K, φ22 to 10.4 mm), wire drawing (φ10.4 to 6 mm) and solution treatment (Al-8Mg alloy: 593 K
16 h), surface cutting (φ30 to 22 mm), hot swage (473 K, φ22 to 10.4 mm), wire drawing (φ10.4 to 6 mm) and solution treatment (Al-8Mg alloy: 593 K 1 h, Al-8Mg-Zr alloy: 798 K
1 h, Al-8Mg-Zr alloy: 798 K 2 h) were conducted. The grain size was about 100 µm in both alloys after solution treatment. The microstructure of the Al-8Mg-Zr alloy was observed using a High-angle annular dark field scanning transmission electron microscopy (HAADF-STEM). Tensile tests were carried out under the conditions at 298 K and 523 K in air and at a strain rate of 1.0
2 h) were conducted. The grain size was about 100 µm in both alloys after solution treatment. The microstructure of the Al-8Mg-Zr alloy was observed using a High-angle annular dark field scanning transmission electron microscopy (HAADF-STEM). Tensile tests were carried out under the conditions at 298 K and 523 K in air and at a strain rate of 1.0 10-3 s-1. After the tensile test, the elemental analysis of the fracture surface was measured using Auger electron spectroscopy (AES) and fracture surfaces were observed with a scanning electron microscopy (SEM). For AES analysis, an Al-8Mg-0.2Zr alloy added 70 mass ppm Si (Low purity (LP)) was used to obtain grain boundary fracture surface.
10-3 s-1. After the tensile test, the elemental analysis of the fracture surface was measured using Auger electron spectroscopy (AES) and fracture surfaces were observed with a scanning electron microscopy (SEM). For AES analysis, an Al-8Mg-0.2Zr alloy added 70 mass ppm Si (Low purity (LP)) was used to obtain grain boundary fracture surface.
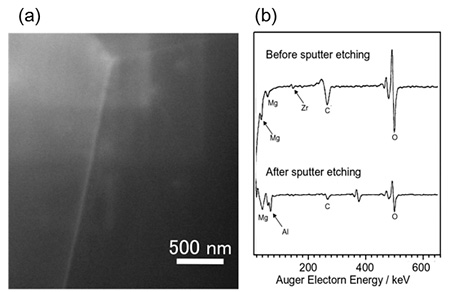
Figure 2 (a) shows HAADF-STEM images of Al-8Mg-0.2Zr alloy. White contrast is confirmed in grain boundary. Since Zr is the element with the largest atomic number among the constituent elements of Al-8Mg-0.2Zr alloy, it is considered that the white contrast corresponds to Zr. Grain boundary segregated element was analyzed using STEM-EDS, but Zr was not detected on the grain boundary. Therefore, grain boundary segregation of Zr was confirmed by other methods.
AES was used for analysis of grain boundary segregated elements. In Al-Mg alloys, intergranular fracture occurs in the high temperature region (473-623 K), and embrittlement is accelerated when Si is included. Therefore, Al-8Mg-0.2Zr alloy (LP) added with 70ppm Si was tensile tested at 523 K and the grain boundary fractured surface was subjected to AES analysis. The AES results before and after sputtering by Ar gas on the fracture surface of Al-8Mg-0.2Zr alloy (LP) are shown in Fig. 2 (b). As shown in Fig. 2 (b), elemental peak of Zr was observed before sputtering, but elemental peak of Zr was not observed after sputtering. From these results, it became clear that Zr segregates at the grain boundary in the Al-8Mg-0.2Zr alloy.
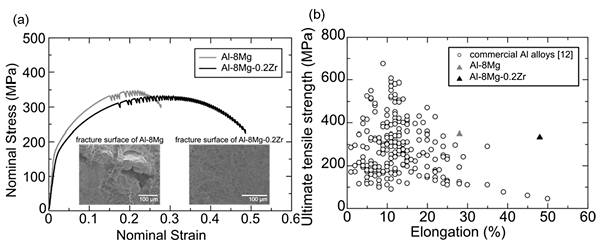
Figure 3(a) shows stress-strain curves and SEM images of fracture surfaces of the Al-8Mg alloy and Al-8Mg-0.2Zr alloy after tensile test at 298 K. As shown in Fig. 3(a), elongation of Al-8Mg alloy was 28%, whereas that of Al-8Mg-0.2Zr alloy was 49%. Intergranular fracture was observed in part of fracture surface in Al-8Mg alloy. In contrast in Al-8Mg-0.2Zr alloy, transgranular fracture was observed on the whole surface and intergranular fracture was suppressed. From the above results, it was revealed that intergranular fracture is suppressed by strengthening the grain boundary of Al-8Mg alloy through adding Zr. Fig. 3(b) shows the relationship between strength and elongation of commercial Al alloys, Al-8Mg alloy and Al-8Mg-0.2Zr alloy. As shown in Fig. 3 (b), it was revealed that addition of Zr suppresses intergranular fracture and improves ductility. By adding Zr to the Al-8Mg alloy, it was possible to develop alloys which greatly exceed the trade-off balancing between strength and ductility of commercial alloys.