Recently, electrodeposition processes for Al and Al alloys using non-aqueous solvents have been developed. Al electrodeposition, which is much easier than conventional molten salt methods, is expected to be applied for surface treatments in micro electromechanical systems, as a negative electrode for lithium batteries, and in aluminum production. The mixture of dimethylsulfone and aluminum chloride (DMSO2 bath) is a common non-aqueous solvent for Al and Al alloy electrodeposition that provides good thermal stability and low toxicity. Electrodeposition using a DMSO2 bath offers a high current efficiency of![]() 100 %. Al electrodeposited using this method has been reported to exhibit a remarkably high hardness due to the formed nanostructured microstructures. However, electrodeposited Al and Al alloys suffer from limited ductility, as Al electrodeposited from a DMSO2 bath fractures in the elastic region during tensile testing. The main cause of this inadequate ductility is contamination by S and Cl, which are impurities that originate from decomposition of the electrolytes in the DMSO2 bath. To improve the ductility of Al and Al alloys electrodeposited from DMSO2 baths, a technique to suppress contamination of both impurities must be developed. In a recent study, the addition of gallium chloride to the DMSO2 bath was shown to reduce impurity content in electrodeposited Al–Ga alloys, resulting in improved plastic deformability. Unfortunately, the tensile elongation of the Al–Ga alloys remained low at
100 %. Al electrodeposited using this method has been reported to exhibit a remarkably high hardness due to the formed nanostructured microstructures. However, electrodeposited Al and Al alloys suffer from limited ductility, as Al electrodeposited from a DMSO2 bath fractures in the elastic region during tensile testing. The main cause of this inadequate ductility is contamination by S and Cl, which are impurities that originate from decomposition of the electrolytes in the DMSO2 bath. To improve the ductility of Al and Al alloys electrodeposited from DMSO2 baths, a technique to suppress contamination of both impurities must be developed. In a recent study, the addition of gallium chloride to the DMSO2 bath was shown to reduce impurity content in electrodeposited Al–Ga alloys, resulting in improved plastic deformability. Unfortunately, the tensile elongation of the Al–Ga alloys remained low at![]() 2 %. Ga negatively influences Al materials by decreasing grain boundary (GB) cohesion. The introduction of other impurities that degrade material performance (such as S and Cl) prevented significant ductility improvement. Therefore, we tested metal salts that do not adversely affect ductility and have the potential to reduce impurity content. Herein, we report the improved tensile elongation of Al containing a trace amount of Sn electrodeposited from a DMSO2 bath doped with tin chloride. The microstructure of the electrodeposited samples was characterized to determine the mechanisms underlying the improvement in tensile elongation.
2 %. Ga negatively influences Al materials by decreasing grain boundary (GB) cohesion. The introduction of other impurities that degrade material performance (such as S and Cl) prevented significant ductility improvement. Therefore, we tested metal salts that do not adversely affect ductility and have the potential to reduce impurity content. Herein, we report the improved tensile elongation of Al containing a trace amount of Sn electrodeposited from a DMSO2 bath doped with tin chloride. The microstructure of the electrodeposited samples was characterized to determine the mechanisms underlying the improvement in tensile elongation.
Preparation of the electrolytes and electrodeposition were performed in an Al-filled glove box. The electrolytes were composed of anhydrous aluminum chloride and DMSO2 at a molar ratio of 3:10. Aluminum chloride was used as-received, while DMSO2 was used after vacuum drying. These reagents were slowly mixed at![]() 110 °C while monitoring heat generation. After the solution clarified, anhydrous tin chloride was added. The amount of tin chloride was 0.0002 mol with respect to 10 mol DMSO2. A copper plate was used as the cathode, while an Al plate was used as the anode. Electrodeposition was performed at a direct current density of 30 mA/cm2 and bath temperature of 110 °C. Tensile specimens with a gauge length of 10.0 mm, width of 4.0 mm, and thickness of 0.4 mm, were machined via electrical discharge machining (EDM). It should be noted that the copper substrate and layer affected by EDM were removed via mechanical polishing. The tensile tests were performed at room temperature at a strain rate of 1
110 °C while monitoring heat generation. After the solution clarified, anhydrous tin chloride was added. The amount of tin chloride was 0.0002 mol with respect to 10 mol DMSO2. A copper plate was used as the cathode, while an Al plate was used as the anode. Electrodeposition was performed at a direct current density of 30 mA/cm2 and bath temperature of 110 °C. Tensile specimens with a gauge length of 10.0 mm, width of 4.0 mm, and thickness of 0.4 mm, were machined via electrical discharge machining (EDM). It should be noted that the copper substrate and layer affected by EDM were removed via mechanical polishing. The tensile tests were performed at room temperature at a strain rate of 1 ![]() 10-3 s-1. To evaluate the hardness of the electrodeposits, micro-Vickers hardness tests were conducted on the surface of the electrodeposited samples using a load of 100 g for 10 s. The microstructure of the electrodeposited samples was characterized using an electron back scatter diffraction system (EBSD) attached to a scanning electron microscope (SEM). The S and Cl contents were measured via electron probe micro analysis (EPMA). Using these characterization methods, the presence of precipitates was confirmed and identified as Sn by EPMA. The Sn content was measured via inductively coupled plasma-mass spectrometry (ICP-MS).
10-3 s-1. To evaluate the hardness of the electrodeposits, micro-Vickers hardness tests were conducted on the surface of the electrodeposited samples using a load of 100 g for 10 s. The microstructure of the electrodeposited samples was characterized using an electron back scatter diffraction system (EBSD) attached to a scanning electron microscope (SEM). The S and Cl contents were measured via electron probe micro analysis (EPMA). Using these characterization methods, the presence of precipitates was confirmed and identified as Sn by EPMA. The Sn content was measured via inductively coupled plasma-mass spectrometry (ICP-MS).
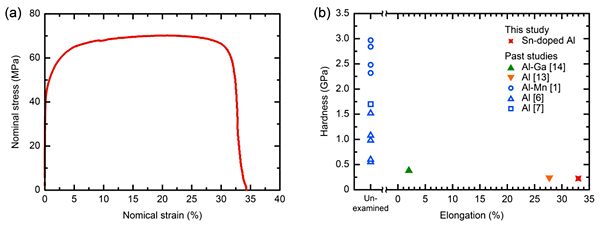
The stress-strain curves of the electrodeposited samples are shown in Fig. 1a. The electrodeposited samples showed a tensile strength of 78 MPa and clear elongation of 33 %. For comparison with previously reported results, the relationship between strength and elongation is summarized in Fig. 1b. The hardness value was adopted as a measure of strength and the values obtained from the samples not subjected to the tensile test was plotted on the unexamined axis. This was to facilitate the comparison because most studies of electrodeposited Al and Al alloys only reported hardness. The reported hardness values were as high as 3.0 GPa. Although these values are very high for Al and Al alloys, the tensile properties of these hard samples were not reported. Samples with hardness values of <0.4 GPa exhibited plastic deformation and the electrodeposited samples in this study exhibited the highest elongation of 33 %.
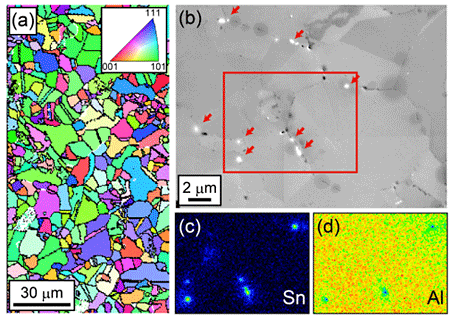
The microstructure of the electrodeposited samples was characterized to identify the cause of the improved elongation. The EBSD image showing the obtained microstructure is shown in Fig. 2a, revealing a grain size in the microcrystalline range with an average grain size of 8.6 µm. In addition, the presence of precipitates was confirmed, as shown in Fig. 2b. The precipitates were identified by EPMA mapping (Figs. 2c and 2d) and determined to be composed of Sn. The EPMA analysis also showed that the S and Cl contents were both <0.1 at%, while subsequent ICP-MS measurement showed that the Sn content was 0.05 at%.
As mentioned in the introduction, DMSO2 baths introduce large amounts of S and Cl impurities (up to ![]() 0.8 at%) in the electrodeposits. Although the location and form of S and Cl were not obvious in the above analyses. The Ga segregation and Sn precipitation at the GBs as shown in Fig. 2d likely reduced the S and Cl contents. We assumed that S and Cl were also present at the GB or in the vicinity in the electrodeposited Al. To reduce the amount of impurities in the electrodeposits, it is important to occupy GBs with preventative materials before the invasion of harmful elements such as S and Cl. Conversely, removing S and Cl lead to a coarsening of grain size to the µm-scale (Fig. 2), resulting in decreased strength, as shown in Fig. 1b. The compatibility of the techniques developed in this study and grain refinement to the nanometer scale will be the subject of future investigations.
0.8 at%) in the electrodeposits. Although the location and form of S and Cl were not obvious in the above analyses. The Ga segregation and Sn precipitation at the GBs as shown in Fig. 2d likely reduced the S and Cl contents. We assumed that S and Cl were also present at the GB or in the vicinity in the electrodeposited Al. To reduce the amount of impurities in the electrodeposits, it is important to occupy GBs with preventative materials before the invasion of harmful elements such as S and Cl. Conversely, removing S and Cl lead to a coarsening of grain size to the µm-scale (Fig. 2), resulting in decreased strength, as shown in Fig. 1b. The compatibility of the techniques developed in this study and grain refinement to the nanometer scale will be the subject of future investigations.
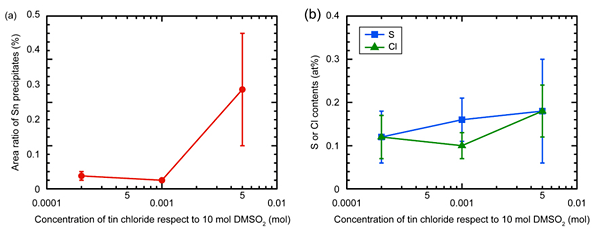
The concentration of tin chloride in the deposition bath was optimized to reduce S and Cl contamination. Electrodeposition was performed with increasing concentrations of tin chloride from 0.0002 to 0.005 mol with respect to 10 mol DMSO2. The area ratio of Sn precipitates as well as S and Cl contents of the electrodeposited samples were plotted as a function of the concentration of tin chloride with respect to 10 mol DMSO2 (Fig. 3). When 0.001–0.05 mol tin chloride was added to the deposition bath, area ratio of Sn precipitates detected using SEM images was ranging from 0.01 to 0.05 % (Fig. 3a). These samples contained reduced S and Cl contents of ![]() 0.1 at% (Fig. 3b). In contrast, Sn was not detected for the sample electrodeposited with 0.0002 mol tin chloride in the bath. This sample containing large amounts of S and Cl (Fig. 3b) similar to that of the conventionally electrodeposited Al. The addition of at least 0.001 mol of tin chloride with respect to 10 mol DMSO2 rendered the electrodeposit impurities harmless. The results of this study indicate the excellent impurity suppressing effect of Sn for Al electrodeposition via DMSO2 baths.
0.1 at% (Fig. 3b). In contrast, Sn was not detected for the sample electrodeposited with 0.0002 mol tin chloride in the bath. This sample containing large amounts of S and Cl (Fig. 3b) similar to that of the conventionally electrodeposited Al. The addition of at least 0.001 mol of tin chloride with respect to 10 mol DMSO2 rendered the electrodeposit impurities harmless. The results of this study indicate the excellent impurity suppressing effect of Sn for Al electrodeposition via DMSO2 baths.