The high productivity and bath stability of electrodeposition for Al and its alloys from a dimethylsulfone (DMSO2)-aluminum chloride bath are highly desirable characteristics for a wide range of critical applications. However, a long-standing problem for such electrodeposits is that they suffer from S and Cl contaminations, resulting in limited ductility. In this study, we explored a reduction method for both S and Cl content in Al electrodeposits from a DMSO2 bath to facilitate plastic elongation of the electrodeposited Al.
Four types of electrolytes were prepared with molar ratios ranging from 2–5:10 AlCl3 to DMSO2. For alloy electrodeposition, metal salts added to the solvent with a molar ratio of 3:10. Eight types of metal salts were utilized: TiCl4, CrCl3, MnCl2, FeCl2, NiCl2, CuCl, ZnCl2, and GaCl3. The amount of the metal salts added was adjusted to 0.01 mol in 10 mol of DMSO2. The S and Cl contents were measured by an energy-dispersive X-ray spectroscope attached to a scanning electron microscope (SEM, Hitachi S-4800). The C content of some samples was quantified via infrared absorption following combustion in a high-frequency induction furnace (LECO CS-LS600). To evaluate the hardness of the electrodeposits, micro-Vickers hardness tests were conducted on the surface of electrodeposited samples utilizing a load of 50–300 g for 10 s. Tensile tests were carried out at room temperature with a strain rate of 1 ![]() 10-3 s-1. The grain boundary (GB) segregation energy and surface segregation energy are calculated from first-principles calculations.
10-3 s-1. The grain boundary (GB) segregation energy and surface segregation energy are calculated from first-principles calculations.
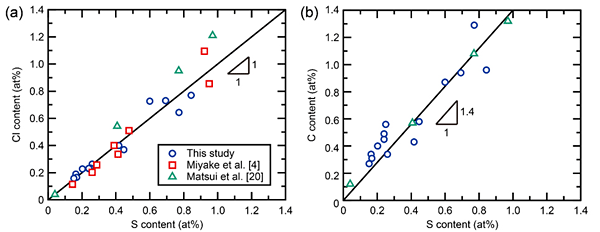
Figure 1 shows the relationships between S content and (a) Cl or (b) C contents in Al electrodeposited from a DMSO2-based bath as obtained from the literature and verified in this study. Both the S and Cl contents of electrodeposited Al range from 0.2 to 0.8 at%. These impurity contents are mainly tied to the agitation speed and molar ratio of AlCl3 to DMSO2 and are not affected by current density or bath temperature. A constant ratio of 1:1 for the S to Cl contents was observed. Based on these results, we speculate that S- and Cl-containing ions form clusters with a ratio of 1:1 in the mixture of AlCl3 and DMSO2.
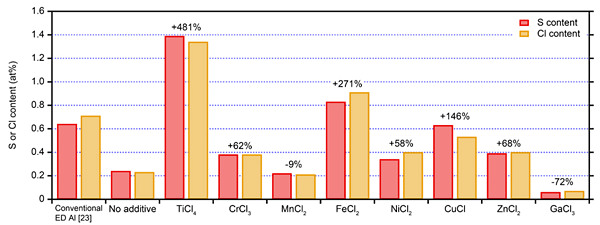
Figure 2 shows the S and Cl contents of Al-X alloys (X = Ti, Cr, Mn, Fe, Ni, Cu, Zn, and Ga) electrodeposited from a DMSO2-based bath at a current density of 30 mA/cm2 and bath temperature of 110 °C, as well as those of electrodeposited Al previous studies and this study. ]The addition of the metal salts, such as TiCl4, CrCl3, MnCl2, FeCl2, NiCl2, CuCl, ZnCl2, and GaCl3, alters the S and Cl contents of electrodeposits. TiCl4 resulted in the highest S and Cl contents of 1.5 and 1.3 at%, respectively, whereas GaCl3 resulted in the lowest S and Cl contents of less than 0.1 at%. These changes in S and Cl contents originate from the interaction between the S- and Cl-containing clusters and the metallic ions. Exploration of these interactions can help us develop more efficient purification methods for electrodeposited Al and Al alloys.
Figure 3 shows EBSD image showing the microstructure of Al-Ga alloys electrodeposited from a DMSO2-based bath and Fig. 4 shows the typical stress-strain curve of electrodeposited Al-Ga alloys. The reduction of S and Cl contents through the addition of GaCl3 resulted in microstructures with a relatively large grain size of 23 µm. Therefore, the overall material strength was lowered compared to conventional electrodeposited Al, but tensile elongation in the electrodeposited Al alloys was confirmed for the first time.
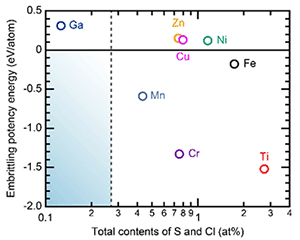
Discussions based on growth modes during electrodeposition revealed the possibility that Ga ions can inhibit growth and the results in defects in electrodeposits. Additionally, as shown in Fig. 5, first-principles calculations based on the segregation energies of solute atoms and discussions based on the Rice–Wang model revealed the embrittling ability of Ga in an Al matrix. This result explained the limited tensile elongation of ![]() 2 % in the electrodeposited Al-Ga alloys. This discussion also provided important guidance for future work to enhance the tensile elongation of electrodeposited Al alloys.
2 % in the electrodeposited Al-Ga alloys. This discussion also provided important guidance for future work to enhance the tensile elongation of electrodeposited Al alloys.