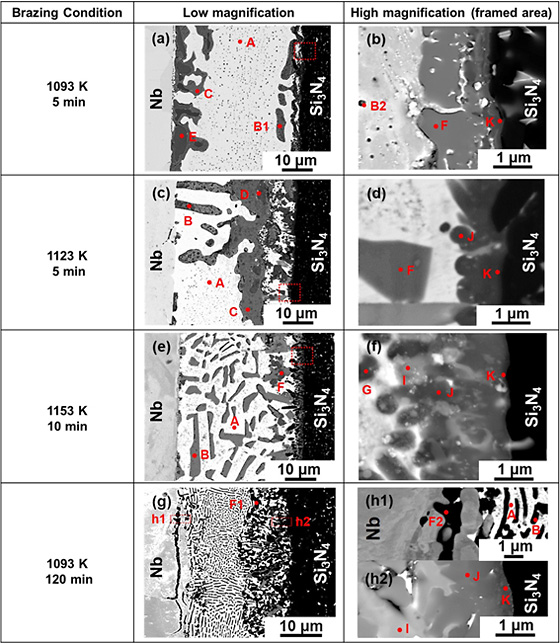
Robust Si3N4/Ti joints with Nb interlayer brazed using 68.8Ag26.7Cu4.5Ti (mass%) filler alloy (TICUSIL®) were developed. The interfacial microstructural evolution and mechanical behavior with respect to various brazing temperatures (1093–1153 K) and holding times (5–120 min) were mainly governed by reaction kinetics of Ti in the filler alloy. Depending on the relative distribution of Ti atoms, types of Cu–Ti intermetallics formed differ across the interface. At Ti/Nb interface, a multi–layered compounds consisted of Ag–solid solution sandwiched in between Cu–Ti intermetallics were formed for all brazing conditions. At Nb/Si3N4 interface, competing Ti reactions were discovered when brazed at 1093 K for 5 min. With rising brazing temperature, increasing preferential reactivity of Ti for Si3N4 instead of Cu minimized the formation of Cu–Ti intermetallics. Besides functioning as a residual stress reliever, Nb inhibited migration of ejected Ti from the substrate toward Si3N4, minimizing intermetallics formation at Nb/Si3N4 interface. All joints fractured at Nb/Si3N4 interface due to residual stress when tested at room temperature. Brazing at 1153 K for 10 min produced the highest average bending strength of 181 MPa. Fracture mechanisms differ depending on both reaction layer thickness and residual stress caused by CuTi.
Interfacial Microstructural Evolution, Diffusion Inhibition and Fracture Behavior
of Nb Interlayer Inserted Si3N4/Ti Joints Brazed with AgCuTi Filler Alloy
[Published in Materials Science & Engineering A, doi.org/10.1016/j.msea.2019.138096]
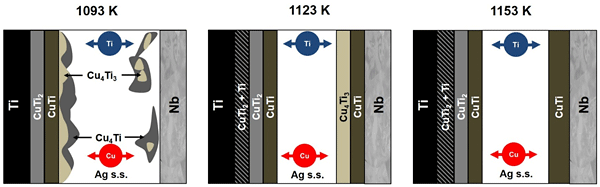 |
| Fig. 1 Schematics of interfacial microstructural evolution and elements diffusion at Ti/Nb interface when brazed with relatively short holding time. |
 |
|
Fig. 2 Low–and high–magnification cross–sectional BSE images of Nb/Si3N4 interfacial microstructure formed at different brazing conditions. Reaction compounds are denoted by: A: Ag s.s., B: Cu s.s., C: Cu4Ti, D: Cu3Ti2, E: Cu4Ti3, F: CuTi, G: CuTi2, I: (Nb, Ti)5Si3, J: Ti5Si3, TiN and K: TiN. |