Anodic porous alumina has been widely used as an oxide coating of Al surfaces. Highly ordered porous alumina is obtained by long-time anodization under appropriate conditions. Typically, hexagonally arranged nanopores with an interpore distance of 100 nm are formed in oxalic acid at 40 V. Here, we describe the formation of finer porous alumina by anodization in a potassium oxalate solution (pH 7.6) at 40 V. We found that the interpore distance is considerably reduced from 100 nm to ![]() 25 nm by anodization in a potassium or sodium oxalate solution. The anodization of Al in a neutral electrolyte (typically ammonium tetraborate) has been used for the formation of barrier oxide films, whose thickness is controlled only by the anodization voltage (1.4 nm/V). In the case of anodization in an oxalate solution, a fine nanoporous film was formed in a wide range of temperatures (0-85 °C) and a nonporous barrier film was not obtained.
25 nm by anodization in a potassium or sodium oxalate solution. The anodization of Al in a neutral electrolyte (typically ammonium tetraborate) has been used for the formation of barrier oxide films, whose thickness is controlled only by the anodization voltage (1.4 nm/V). In the case of anodization in an oxalate solution, a fine nanoporous film was formed in a wide range of temperatures (0-85 °C) and a nonporous barrier film was not obtained.
An Al sheet (99.99% purity, 0.4 or 0.1 mm thickness, Toyo Aluminium K.K., Japan) was cut to 1.5 ![]() 3.5 cm2. Anodization was conducted in 0.20 M potassium oxalate in a 500 ml beaker with a magnetic stirrer using a three-electrode system with a potentiostat (Hokuto Denko HA-501S). Here, all the potentials are given with respect to the Hg/Hg2SO4 electrode in saturated K2SO4 (BAS RE-2C, +0.64 V vs SHE at 25 °C). A large Al sheet was used as the counter electrode.
3.5 cm2. Anodization was conducted in 0.20 M potassium oxalate in a 500 ml beaker with a magnetic stirrer using a three-electrode system with a potentiostat (Hokuto Denko HA-501S). Here, all the potentials are given with respect to the Hg/Hg2SO4 electrode in saturated K2SO4 (BAS RE-2C, +0.64 V vs SHE at 25 °C). A large Al sheet was used as the counter electrode.
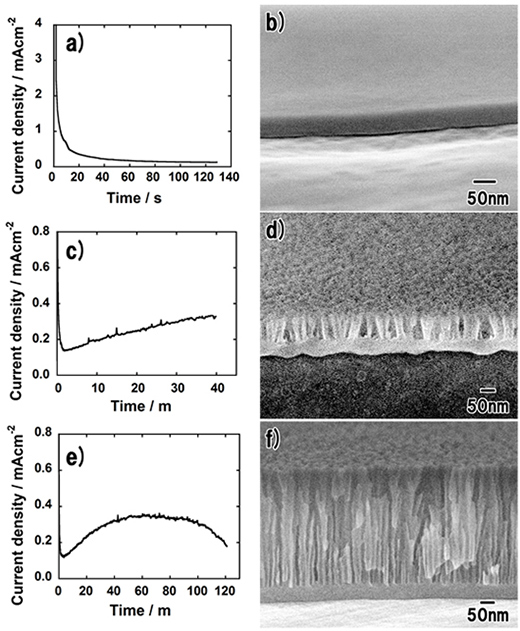
Figure 1 shows the results of Al anodization in 0.20 M potassium oxalate at 40 V for 6 h at 25 °C. Figure 1a shows that the current gradually increased to ![]() 0.4 mAcm-2 in 40 min then gradually decreased. After anodization for 2 h, the current reached a low value and remained at
0.4 mAcm-2 in 40 min then gradually decreased. After anodization for 2 h, the current reached a low value and remained at ![]() 0.1 mAcm-2. Figure 1b shows a cross-sectional SEM image of the obtained oxide film. Since the interpore distance of anodic porous alumina formed in conventional oxalic acid at 40 V is 100 nm, the image clearly indicates that the pores are unusually fine. It is also confirmed that the oxide film is composed of following three layers: an upper porous layer that contains large pores, a lower porous layer with fine and straight pores and a flat barrier layer with a thickness of
0.1 mAcm-2. Figure 1b shows a cross-sectional SEM image of the obtained oxide film. Since the interpore distance of anodic porous alumina formed in conventional oxalic acid at 40 V is 100 nm, the image clearly indicates that the pores are unusually fine. It is also confirmed that the oxide film is composed of following three layers: an upper porous layer that contains large pores, a lower porous layer with fine and straight pores and a flat barrier layer with a thickness of ![]() 60 nm. The total thickness of the oxide film is 440 nm.
60 nm. The total thickness of the oxide film is 440 nm.
To investigate the growth behavior of fine pores, anodization was terminated at each characteristic point of the anodic current: the first decrease prior to a peak, the peak and after the second decrease. Figures 2a and 2b show the anodic current and cross-sectional SEM image obtained after anodization for 130 s, respectively. As shown in Fig. 2a, anodization was terminated when the anodic current reached the low value. Figure 2b shows that a flat barrier film without fine pores was formed. The thickness of the film was ![]() 50 nm. Figures 2c and 2d show the anodic current and cross-sectional SEM image obtained after anodization for 40 min, respectively. In this case, anodization was terminated when the anodic current reached maximum (Fig. 2c). It is confirmed from Fig. 2d that fine pores with a depth of
50 nm. Figures 2c and 2d show the anodic current and cross-sectional SEM image obtained after anodization for 40 min, respectively. In this case, anodization was terminated when the anodic current reached maximum (Fig. 2c). It is confirmed from Fig. 2d that fine pores with a depth of ![]() 60 nm emerged in the oxide film. At the maximum of the anodic current, larger pores started to dominate the growth of the film because the barrier layer was deformed to become concave at the bottom of each larger pore. This behavior is identical to that occurring in conventional oxalic acid, sulfuric acid or phosphoric acid, where small pores at the surface are left behind and larger pores continue growing to form a thick porous film. However, all the pores are fine in the case of anodization in potassium oxalate. Figures 2e and 2f show the anodic current and cross-sectional SEM image obtained after anodization for 120 min, respectively, after the anodic current had decreased from the peak (Fig. 2e). The structure of the porous film (Fig. 2f) is almost identical to that obtained by anodization for 6 h (Fig. 1b). Therefore, the growth of nanopores terminates when the anodic current decreases from its peak to a low value. These results mean that the larger pores formed at the early stage of the anodization cannot maintain their growth throughout the anodization. This growth behavior is different from that of the existing porous films.
60 nm emerged in the oxide film. At the maximum of the anodic current, larger pores started to dominate the growth of the film because the barrier layer was deformed to become concave at the bottom of each larger pore. This behavior is identical to that occurring in conventional oxalic acid, sulfuric acid or phosphoric acid, where small pores at the surface are left behind and larger pores continue growing to form a thick porous film. However, all the pores are fine in the case of anodization in potassium oxalate. Figures 2e and 2f show the anodic current and cross-sectional SEM image obtained after anodization for 120 min, respectively, after the anodic current had decreased from the peak (Fig. 2e). The structure of the porous film (Fig. 2f) is almost identical to that obtained by anodization for 6 h (Fig. 1b). Therefore, the growth of nanopores terminates when the anodic current decreases from its peak to a low value. These results mean that the larger pores formed at the early stage of the anodization cannot maintain their growth throughout the anodization. This growth behavior is different from that of the existing porous films.
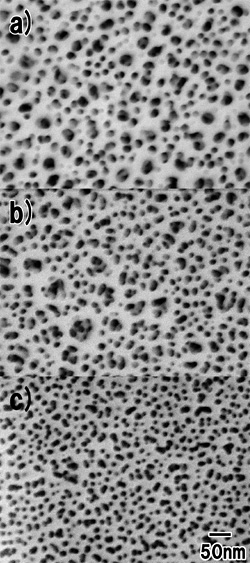
To further investigate the fine pores inside the oxide film, the surface of the porous film was ion-milled. Figure 3a shows a surface SEM image of the porous alumina before ion milling. The uppermost surface is composed of large (![]() 30 nm) and fine (
30 nm) and fine (![]() 10 nm) pores, both randomly distributed on the surface. Figure 3b shows a surface SEM image of the porous alumina in the upper half of the porous layer. While the size of the small pores did not change, that of the large pores increased. Furthermore, fine pores were observed at the bottom of each large pore. This means that the large pores branch to form small pores during the potentiostatic anodization. In the lower part of the porous film in Fig. 3c, only fine pores existed in the film. From Fig. 3c, the average interpore distance was estimated to be
10 nm) pores, both randomly distributed on the surface. Figure 3b shows a surface SEM image of the porous alumina in the upper half of the porous layer. While the size of the small pores did not change, that of the large pores increased. Furthermore, fine pores were observed at the bottom of each large pore. This means that the large pores branch to form small pores during the potentiostatic anodization. In the lower part of the porous film in Fig. 3c, only fine pores existed in the film. From Fig. 3c, the average interpore distance was estimated to be ![]() 25 nm.
25 nm.
In the case of anodization in a borate solution, fine pores grow on the barrier film only in a hot electrolyte (above ![]() 60 °C). However, in the case of anodization in potassium oxalate, fine pores are obtained in a wide range of temperatures. Figure 4a shows a cross-sectional SEM image of anodic porous alumina obtained by anodization at 0 °C for 27 h. Although the anodic current did not decrease within 27 h, fine pores were formed in the lower porous layer. The growth rate of the film was estimated to be 0.5 nm/min. When Al was anodized at 85 °C, the growth rate of the porous film considerably increased to 170 nm/min (Fig. 4b). Although thick porous alumina with a thickness of 2.5 µm was obtained, the chemical dissolution of the film proceeded. The collapse of the pores in the middle of the porous layer was probably caused by the branching of large pores to form fine pores, deteriorating the arrangement of the pores.
60 °C). However, in the case of anodization in potassium oxalate, fine pores are obtained in a wide range of temperatures. Figure 4a shows a cross-sectional SEM image of anodic porous alumina obtained by anodization at 0 °C for 27 h. Although the anodic current did not decrease within 27 h, fine pores were formed in the lower porous layer. The growth rate of the film was estimated to be 0.5 nm/min. When Al was anodized at 85 °C, the growth rate of the porous film considerably increased to 170 nm/min (Fig. 4b). Although thick porous alumina with a thickness of 2.5 µm was obtained, the chemical dissolution of the film proceeded. The collapse of the pores in the middle of the porous layer was probably caused by the branching of large pores to form fine pores, deteriorating the arrangement of the pores.
The formation mechanism of the unusually fine pores in the neutral oxalate solution appears to be extremely complicated and we cannot explain it at present. Note that the fine porous structure was also formed in sodium oxalate but not in ammonium oxalate even when the pH was increased using NH3. We expect that the clarification of the mechanism will lead to the formation of ultimately miniaturized pores and that ultrafine porous alumina can be utilized similarly to mesoporous silica.
The authors thank Prof. Hidetaka Asoh for the GD-OES measurements of the samples and Toyo Aluminium KK for providing the Al sheets. The authors acknowledge the financial support of The Light Metal Educational Foundation Fellowship Grant.