In our previous study, we successfully formed porous alumina films by indirect oxidation under an alternating current (AC) electric field without a direct electrical connection (“AC-bipolar anodization”). Our approach has the following features: i) the aluminum can be oxidized indirectly in aqueous solutions via wireless treatment under an AC electric field; ii) AC electrolysis is not used to form porous alumina films with a thickness and pore diameter gradient but to form uniform coatings; and iii) numerous small objects can be treated simultaneously. More importantly, despite the indirect wireless oxidation, porous alumina films grew uniformly on aluminum balls. Although we believe that these results open a new route to technological and scientific applications of aluminum-based products, the fundamental understanding of indirect oxidation under an AC electric field remains insufficient.
In the present study, we continued our preliminary work and focused on the differences between the use of DC and AC electric fields to obtain a better understanding of the effect of an external electric field and frequency on oxide film formation on an unconnected aluminum electrode. The thickness and uniformity of the films formed by AC-bipolar anodization were evaluated using a spectrophotometric method for dyed specimens and field-emission scanning electron microscopy (FE-SEM). Moreover, the dependence of frequency on film thickness was also investigated by comparison to that of conventional AC anodization.
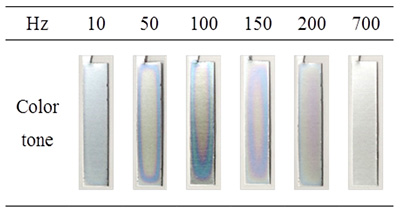
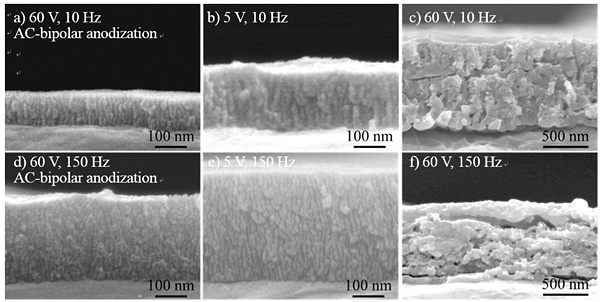
The formation of porous alumina films using bipolar electrochemistry under AC electric fields without a direct electrical connection (“wireless treatment”) was investigated. The effect of frequency on the thickness of the films was evaluated using spectrophotometric analysis of dyed specimens and field-emission scanning electron microscopy. A film formation was simultaneously achieved on both sides of a high-purity aluminum electrode in oxalic acid under an AC electric field. A film formed at 150 Hz was thicker than those formed at other frequencies even in the same reaction time, as shown in Fig. 1. Figure 2 shows cross sectional SEM images of the alumina films formed by AC-bipolar anodization at 60 V. SEM images of the films formed by conventional AC anodization at 5 V and 60 V are displayed for comparison. Although the growth of non-uniform films was observed for AC-bipolar anodization, the morphology of the obtained films was roughly the same as those formed using conventional AC anodization with a direct connection, suggesting a similar oxide formation mechanism. Moreover, the dependence of film thickness on frequency was almost in agreement with the tendency observed for conventional AC anodization reported previously. The main disadvantage of AC-bipolar anodization lies in the decrease of the efficiency of film formation. From the application standpoint, however, the wireless treatment can introduce new possibilities for electrochemical surface treatment of small conductive materials that require a specific manipulation for a direct electrical connection.