In addition to the mechanical properties of biomaterials, chemical and biological properties, particularly surface geometry and topography, play important roles in modulating cellular phenotypes, such as attachment, proliferation, and differentiation. To clarify the effects of grid topographies with different scales on cell morphology and functionalization, we investigated the adhesion and differentiation of human mesenchymal stem cells (hMSCs) to titanium (Ti) surfaces with micron, nano, and micron/nano (hybrid) grid topographies created by femtosecond laser irradiation.
Commercially pure Ti discs with a mirror-polished surface (grade 2, henceforth referred to as Mirror) were purchased and used directly for surface scanning with femtosecond laser irradiation using an FCPA µjewel D-1000 laser (IMRA America Inc., Ann Arbor, MI, USA). To fabricate surface topographies with different scales, the polarization beam was changed according to the processing purpose. To prepare micrometer-scale grid topography on Ti (Micron), parallel grooves with different scales were formed and then the specimen was turned 90° for another scan. Circular polarization was used to scan repeatedly until the desired depth (![]() 1 µm) was achieved. To obtain nanometer-scale grids on Ti (Nano), linear polarizationwas applied in a single scanning. To fabricate micron/nanometer scaled grids (Hybrid), both polarization beams were used and fabrication was performed in two steps, with micron grids formed first followed by overwriting with nano grids.
1 µm) was achieved. To obtain nanometer-scale grids on Ti (Nano), linear polarizationwas applied in a single scanning. To fabricate micron/nanometer scaled grids (Hybrid), both polarization beams were used and fabrication was performed in two steps, with micron grids formed first followed by overwriting with nano grids.
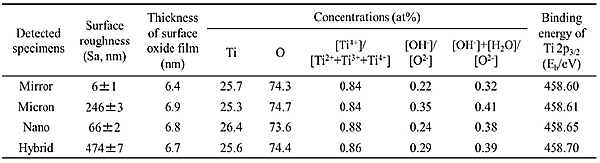
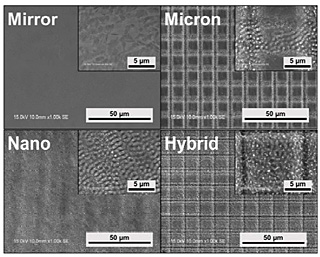
The surface topography of each specimen was characterized with scanning electron microscopy (Fig. 1). Mirror displayed a smooth surface before laser irradiation. Micron displayed cross-groove morphology with the 5-µm wide and 1-µm deep grooves separated by 6-µm ridges. In addition, a nano-ripple structure on the surface of the grooves was formed during the fabrication of the micron grooves by the initial circularly polarized beam. Nano specimens displayed nano grids with a depth of approximately 200 nm that formed after a single laser scanning. Hybrid specimens displayed a combined topography of the Micron and Nano conditions. In addition, the surface roughness (Sa) was calculated with the graphical results, that revealed larger surface roughness with Micron and Hybrid as compared to Nano and Mirror (Table 1).
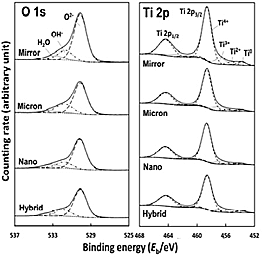
Specimen surface composition and chemical states of Ti alloys with and without laser processing were characterized by X-ray photoelectron spectroscopy (XPS). Relative concentrations of elements and thickness of the surface oxide film were calculated based on XPS spectrum of O 1s and Ti 2p (Fig. 2). Empirical data on relative photoionization cross-sections, σij/σO1s, as 1.28 were used for quantification, where σij/σO1s represented a level j electron (Ti 2p3/2) of an element i (Ti 2p) relative to that of O 1s electrons. As a result, although slightly thicker surface oxide films were observed on Ti specimens after laser processing compared with Mirror (Table 1), this laser irradiation processing increased oxidation was low and did not influence the cell culture results, while a slight progression of oxidation resulted from laser processing.
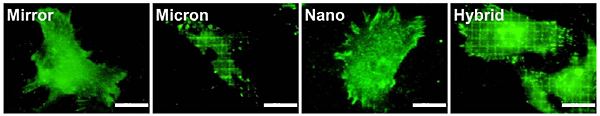
The cellular adhesion behavior on Ti specimen with and without distinct topographies was visualized by immunofluorescent staining. Interestingly, after 24-h incubation, the distribution of adhesion plaque varied according to surface topography (Fig. 3). A clear grid-shaped distribution of vinculin was evident in hMSCs cultured on Micron and Hybrid. In contrast, a filamentous vinculin was displayed at the filopodia of hMSCs cultured on Nano and Hybrid. In addition, Vcl expression was evaluated with real-time RT-PCR (not shown here). Grid topographies promoted the Vcl expression 6 h after cell attachment. In particular, Vcl expression was highest in hMSCs cultured on Hybrid. Our findings indicated that grid topographies strongly promoted cell adhesion. Particularly, the hybrid was effective for cell adhesion compared to the other surface topographies.
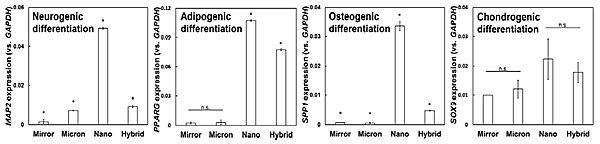
The expression level of selected target genes for neurocyte (MAP2), adipocyte (PPARG), osteocyte (SPP1), and chondrocyte (SOX9) differentiation was assessed by real-time RT-PCR analysis (Fig. 5). After 3D differentiation induction a similar expression level was detected by hMSCs cultured on both Mirror and Micron for adipogenic and chondrogenic differentiation. However, cells cultured on specimens with distinct grid topographies expressed relatively higher levels of target genes compared with those cultured on Mirror. Expression of the target genes differed with the different grid scales, with the differentiation of hMSCs to all four lineages promoted most strongly by Nano. In particular, Spp1 was markedly upregulated in hMSCs cultured on Nano relative to the other specimens. These results indicated that at the early stage of differentiation induction, the Ti surface with nano grid topography strongly stimulated osteogenic differentiation.
In this study, adhesion and differentiation of hMSCs on Ti surfaces with micron, nano, and micro/nano hybrid grid topologies created using femtosecond laser irradiation was evaluated. The results showed that cellular adhesion and differentiation strongly depended on the scales of grid topography. Concerning cell adhesion, the micron-scale topography is beneficial for cell anchoring, while the nanometer-scale topography is beneficial for cell locomotion on the substrate. The micro/nano hybrid grid topography strongly promoted cell adhesion. Ti surfaces with designed grid topographies modulated multilineage differentiation, with osteogenic differentiation being strongly promoted by the nano grid topography. Our results provide a basis for the design of novel biomaterial-cell interfaces that specifically regulate cellular functions.