Almost all dental implants are made of Ti and Ti alloys. This is because Ti exhibits excellent bone compatibility, as represented by osseointegration. However, the dental implants pose a risk of infection, and serious infections associated with bone resorption, known as peri-implantitis. This is a critical problem because it can cause biological failure more than a year after implantation. Decontamination of implants is essential for treatments of peri-implantitis because it is necessary to remove and kill pathogenic bacteria adhering to the surfaces of implants. Coating dental implants with photocatalytically active TiO2 is one approach to making dental implants antibacterially active. Visible-light-active TiO2 coatings can be used for long periods in the treatment of peri-implantitis without having harmful effects on surrounding tissues because such coatings can kill adhered bacteria when and where the implants are exposed to visible light in the mouth. In this study, TiO2 layers were formed on Ti-Au alloys by air oxidation, the Au introduced into the layers was characterized, and tests for stearic acid degradation, antibacterial activity against Escherichia coli (E. coli), and radical formation under visible-light irradiation were conducted.
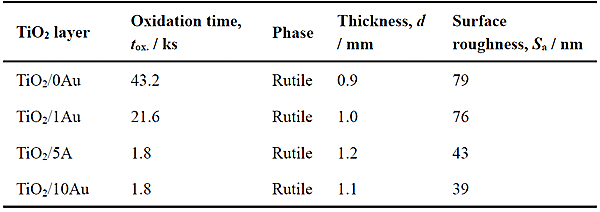
Ti-(0, 1, 5, 10)at%Au alloy plates with mirror-polished surface were used as substrates. These substrates are referred to as 0Au, 1Au, 5Au, and 10Au, respectively. The substrates were air-oxidized at 873 K in the muffle furnace. The oxidation time was maintained within the range of 1.8–43.2 ks to produce the same TiO2 layer thickness on each substrate (approximately 1 µm). The TiO2 layers formed on xAu substrates by air oxidation at 873 K are referred to hereinafter using the nomenclature TiO2/xAu. The synthesis conditions, phases, thicknesses, and arithmetic average heights (surface roughness measures) of the TiO2/0Au, TiO2/1Au, TiO2/5Au, and TiO2/10Au specimens are listed in Table 1. The phase of TiO2 was rutile, independent of the Au contents of the substrates. The arithmetic average height of TiO2/0Au was the greatest at ≈ 80 nm, while that of TiO2/10Au was the smallest at ≈ 40 nm.
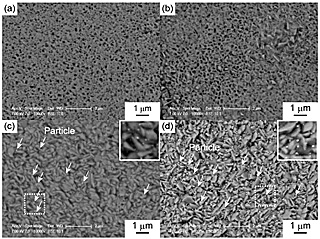
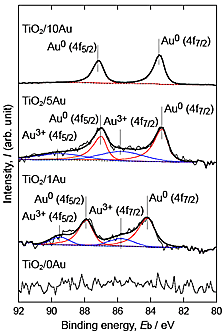
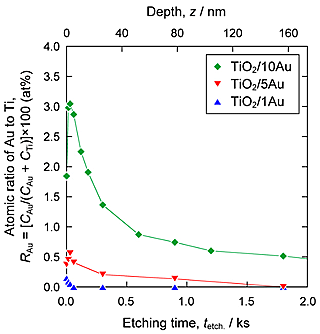
Figure 1 shows SEM images of the surfaces of the TiO2 layers. Nanoparticles were observed on the surfaces of TiO2/5Au and TiO2/10Au, whereas no such particles were observed on the surfaces of TiO2/0Au and TiO2/1Au. Figure 2 shows the Au 4f XPS spectra of the surfaces of the TiO2 layers. Peaks related to Au were detected from TiO2/1Au, TiO2/5Au, and TiO2/10Au. Au0 peaks were detected and attributed to metallic Au. For TiO2/1Au and TiO2/5Au, Au3+ peaks were also detected. Figure 3 shows XPS depth profiles of the atomic ratio of Au to Ti (RAu) in the TiO2 layers on the Ti-Au alloys. RAu was maximized near the surfaces of the TiO2 layers and decreased with layer thickness. The RAu values increased as the Au contents in the substrate increased. From the TEM observations of the TiO2 layers, dark nanoparticles were observed both on the surface and inside of the layer formed on TiO2/5Au. The diameters of the nanoparticles were less than 30 nm. From these results, we concluded that the nanoparticles were metallic Au nanoparticles. This conclusion is consistent with the detection of Au0 in the XPS analysis.
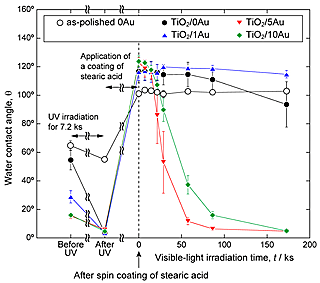
Photocatalytic decomposition of organic compounds on TiO2 layers under visible-light irradiation was evaluated by studying changes in water contact angles. A method modified from JIS R 1753: 2013, evaluation of self-cleaning properties under visible-light irradiation, was adopted in this study, with stearic acid used as a model of an organic compound. Figure 4 illustrates the changes observed in the water contact angles of stearic acid-coated TiO2 layers and as-polished 0Au under visible-light irradiation. The water contact angles of the TiO2 layers decreased to ≈ 5° after cleaning, whereas those of as-polished 0Au decreased only slightly to 55°. Water contact angles were increased to ≈ 120° by the application of a coating of stearic acid to the TiO2 layers. On TiO2/5Au and TiO2/10Au, water contact angles decreased to ≈ 5° under visible-light irradiation for 172.8 ks. On the other hand, on as-polished 0Au and TiO2/1Au, water contact angles did not change during visible-light irradiation. On TiO2/0Au, the decrease in the water contact angle was not remarkable in comparison to that observed for TiO2/5Au and TiO2/10Au. Consequently, TiO2/5Au and TiO2/10Au were concluded to have the highest visible-light responsiveness and experience the greatest stearic acid coating degradation.
The antibacterial activities of the TiO2 layers were evaluated under visible-light irradiation by glass adhesion testing using E. coli. In this study, the protocol was optimized to evaluate specimens of smaller size (Φ12 mm) than those used in testing according to the JIS R 1752: 2013 and ISO 17094: 2014 standards.
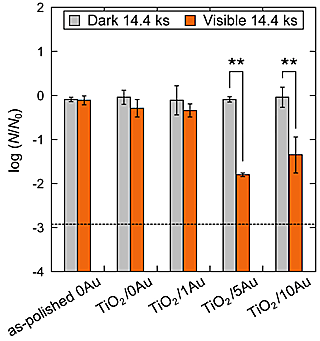
Figure 5 shows the common logarithms of the normalized numbers of viable bacteria after visible-light irradiation and placement in the dark for 14.4 ks, respectively. The dashed line in Fig. 5 indicates the lower detection limit (log(N/N0) = -2.92) under the conditions of this study. For TiO2/5Au and TiO2/10Au, the normalized numbers of viable bacteria after visible-light irradiation decreased and were significantly different (p < 0.01) from those observed after placement in the dark. From the ESR analysis of TiO2/5Au using DMPO (5,5- Dimethyl-1-pyrroline N-oxide), DMPO-OH peaks were detected. As Eq. (1) shows, DMPO-OH is formed by DMPO trapping hydroxyl radicals, •OH, and the test results revealed that •OH was formed by TiO2/5Au under visible-light irradiation.
| •OH + DMPO → DMPO-OH | (1) |