There has been growing interest in the preparation of TiO2 hollow spheres with nanoporous structures owing to their potential applications in various fields, such as photocatalysis, solar cells, and gas sensors. Although a number of methods for the preparation of TiO2 hollow spheres have been developed, it is difficult to prepare porous hollow spheres with controlled geometrical structures on their surface. In our previous report, we described the fabrication of alumina hollow spheres with nanoporous structures on their surface by the anodization of small Al particles. In this process, the anodization of small close-packed Al particles in an acidic electrolyte yields a porous alumina layer on their surface, and the subsequent leaching of the residual Al using an etchant generates hollow spheres with an anodic porous alumina shell. One important advantage of such a process is that the geometrical structures of porous layers can be controlled by adjusting the anodization conditions. In the present report, we describe the preparation of TiO2 hollow spheres with porous structures by the anodization of small Ti particles.
For the preparation of TiO2 porous spheres, Ti particles (average diameter: 92 µm, 99.5% purity) were used as a starting material. Prior to their anodization, the Ti particles were dipped in commercially available chemical etching solution (TCP-80; Mitsubishi Gas Chemical Co.) at 90 °C for 1 min to remove the air-formed oxide layer on their surface. The Ti particles were anodized by a similar process to that reported previously. The Ti particles were packed in a holder, the bottom of which was equipped with a filter membrane with 100 nm pores to enable the circulation of the electrolyte, and then a Ti electrode was set on top of the packed Ti particles under an appropriate pressure to ensure the electrical interconnection between the particles. Pressure was usually applied by a mass of 5 kg/cm2. The anodization of Ti particles was carried out using a mixture of 0.5 wt% HF and 1 M phosphoric acid solution at a constant voltage of 5 - 20 V. After the anodization, the TiO2/Ti particles were mechanically separated. To form the hollow spheres, the residual Ti was dissolved in a saturated iodine methanol solution at 50 °C for 24 h.
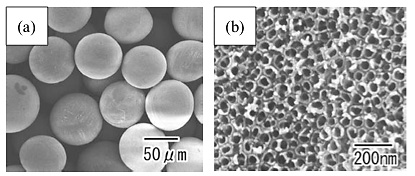
Figure 1 shows SEM images of the Ti particles after anodization at a constant voltage of 10 V for 18 h. From the low-magnification image shown in Fig. 1(a), the formation of the porous layer on the surface of the Ti particles was confirmed. From the high-magnification image shown in Fig. 1(b), it was observed that nanoporous structures were formed on the surface of the Ti particles. The interval and diameter of the holes in the sample in Fig. 1 were 70 and 50 nm, respectively.
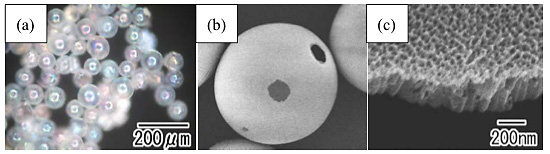
Figure 2 shows an optical microscope image and SEM images of the TiO2 hollow spheres after the leaching of the residual metal. In the close-packed Ti particles, an oxide layer did not form where the Ti particles were in contact because such area did not come in contact with the electrolyte, producing non-oxidized areas on the particles. The residual Ti could be dissolved selectively from the non-oxidized areas. As shown in the optical microscope image in Fig. 2(a), transparent TiO2 hollow spheres were observed. The spherical structures could be maintained even after the removal of the inner residual metal. The rainbow color of the spheres originated from the interference of the incident light at their surface, reflecting the high refractive index of TiO2. In the low-magnification SEM image shown in Fig. 2(b), micron-size openings, which corresponded to the non-oxidized areas, were observed on the surface of each hollow sphere. As shown in the high-magnification SEM image in Fig. 2(c), uniform-size straight holes grew perpendicular to the surface. The thickness of the TiO2 hollow spheres was ca. 500 nm.
Figure 3 shows TiO2 hollow spheres with different hole intervals obtained by varying the anodization voltage between 5 and 20 V. One of advantages of the present process is the controllability of the geometrical structures on the surface of the obtained TiO2 hollow spheres. From the surface SEM images, it can be observed that the hole interval of the porous structures was dependent on the anodization voltage. Figure 3(e) summarizes the relationship between the hole interval and the anodization voltage. From this figure, it was confirmed that the hole interval of the porous structures had an almost linear relationship with the anodization voltage.
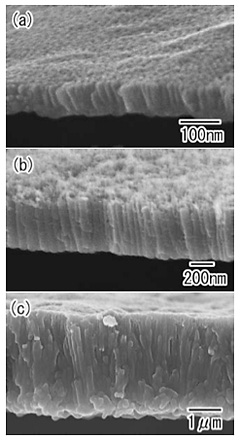
Figure 4 shows TiO2 hollow spheres with different thicknesses of the porous layer. The samples were prepared with anodization times of 4, 30, and 96 h. From the SEM images, it was confirmed that the thickness of the porous layer increased with the anodization time, showing a good linear relationship.