Hydrogen evolution reaction and its analysis on aluminum in sulfuric acid
Osami SERI *,Yuji HOSOI ** and Daichi SASAKI **
*:Department of Mechanical System Engineering , Muroran Institute of Technology
**: Graduate student of Muroran Institute of Technology
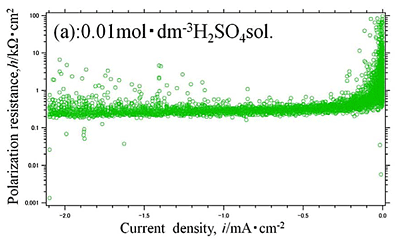
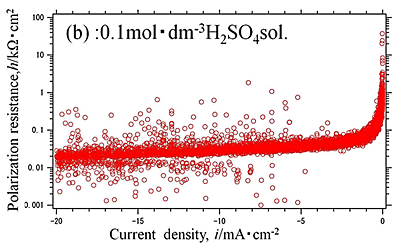
Efforts by trial and error between experimental measurements and theoretical approaches have been made to obtain electrochemical parameters of hydrogen evolution reaction occurred on aluminum in sulfuric acid solution. Polarization curves of aluminum in 0.01, 0.1 and 0.5 mol dm-3 sulfuric acid solutions with dissolved free-oxygen and stagnant conditions were measured and analyzed by the polarization resistance method. They are shown in Fig.1 and Fig.2(a), (b), (c), respectively. It is shown that experimental data of Fig.2(b) was well agree with the theoretical approximate curve (Fig.3). The method reveals that the electrochemical characteristics of hydrogen evolution reaction on aluminum in sulfuric acid solution had the exchanged current density of i0=10-4.5 mA cm-2 in 0.5 mol dm-3 sulfuric acid solution, which generally corresponded with published data elsewhere.
[Published in J. Japan Inst. Metals, Vol. 63 ,No.3(2013), pp.95-100.]
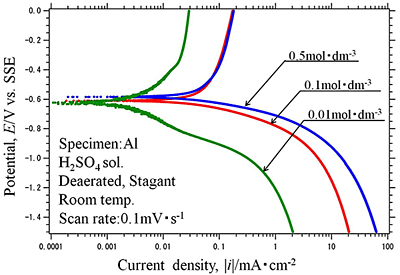 |
| Fig.1 Polarization curves of Al specimen in deaerated H2SO4 solutions at 0.01, 0.1 and 0.5 mol dm-3 |
 |
 |
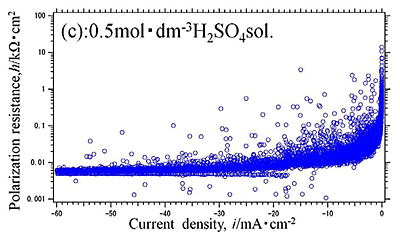 |
| Fig.2 (a), (b), (c) Polarization resistance -current density curve of Al specimen in (a) 0.01, (b) 0.1, (c) 0.5 mol dm-3 |
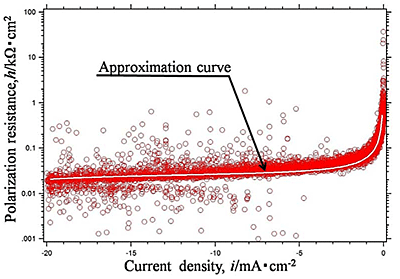 |
| Fig.3 Superimpositions of approximate curves and the experimental curve of 0.1 mol dm-3 H2SO4 solution (Fig.2 (b)) |




