A direct way of NiO reduction by using molten salt electrolysis was investigated to compare with TiO2. The principle of OS reduction process is as follows; The metal oxides such as NiO and TiO2 are immersed into a bath of molten salt and electrolyzed by applying electric field. The metallic products are formed at the cathode and the carbonic gases are expelled at the anode. CaCl2-based salt is taken because it can dissolve up to 20 mol% CaO and because a small addition of CaO in CaCl2 was found to improve the rate of reduction and deoxidation.
Calcium chloride can dissolve 2 to 4 mol% Ca at 1173 K. The presence of Ca dissolved in molten salt is responsible for reduction. The dissolved Ca reacts with the oxide to produce its metal and CaO. When an electric field is applied between the two electrodes, the electrolysis of CaO occurs to produce Ca near the cathode and at the same time two carbonic gases are produced at the anode. CaO becomes the source of Ca for the calciothermic reduction of metal oxide in molten CaCl2 and for the further deoxidation, producing a highly reduced metal with low residual oxygen. This process was effective for TiO2 reduction.
This work aims to examine the CaO concentration that is favourable for reduction and deoxidation of metal oxide in the case of electrolysis in the molten CaCl2 bath. In TiO2 reduction, the complex oxides such as CaTiO3 and Ca2TiO4 were often formed as the intermediate phases during reduction to pure metallic titanium, while NiO did not form any complex oxides, alloys or intermetallic compounds with calcium when NiO was reduced in molten CaCl2. Therefore, NiO was chosen as the example oxide to examine the details of OS process.
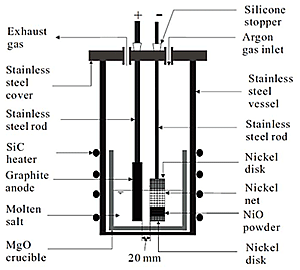
600 grams of CaCl2 was mixed with CaO, and melted in a MgO crucible (Fig. 1). A basket-like Ni cathode contained 1.5g of NiO and it was connected to a stainless steel cathode. A graphite rod was used as the anode. At 1173K the electrodes were submerged into the salt bath, and a constant voltage of 3.1V was applied. After a certain time, they were pulled up to the upper part of the vessel, and the furnace was cooled- down to room temperature. The slightly sintered sample was taken out and sliced into 3 parts. These parts were separately washed, crushed and analyzed.
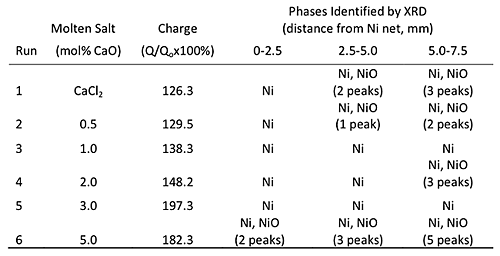
The theoretical charge, Qo, is defined as the charge required to generate stoichiometric amount of Ca reductant for the reduction of the filled amount of NiO. The supplied charge, Q, was calculated by integrating current with respect to time. The ratio of Q/Qo is used as normalized parameter for the supplied electric charge. Table 1 listed the calculated Q/Qo at various CaO concentrations electrolyzed for 1.8 ks at 1173 K. After electrolysis, the supplied charge already exceeded 100% for all experiments. Q/Qo increased with increasing CaO concentration, denoting that the oxide ion transfer is enhanced at higher CaO concentration. Increase of Q/Qo indicates that the larger amount of Ca per time was produced at the higher CaO concentration.
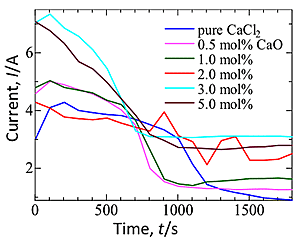
Time dependency of current is shown in Fig. 2. High initial current peaks were observed in the electrolysis at various CaO concentrations, and they were higher at the higher CaO concentration.
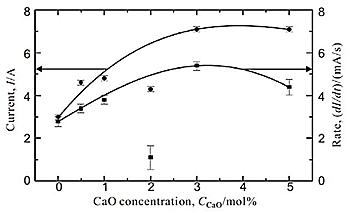
Fig. 3 graphed the current measured for 5 s after the start of electrolysis. This current increased, as CaO was added to the electrolyte until it reaches a plateau at 3.0- 5.0 mol% CaO. Obviously, the increase in onset current with the increase in CaO concentration means that the larger amount of Ca species are being generated in the electrolyte with the higher CaO concentration. Fig. 3 also shows the rate of current decline as a function of CaO concentration. It increased as CaO was added to the electrolyte up to 3.0 mol%, and slightly dropped when CaO content increased to 5.0 mol%. This implies that the rate of reduction was faster at the higher CaO concentration, probably because the larger amount of CaO in the melt was consumed for electrolysis and the larger amount of Ca for reduction is being generated in a short period. Slight decrease in the rate of current decline at 5.0 mol% CaO may be due to the possible precipitation of liquid Ca metal because molten CaCl2 can only dissolved up to 4 mol% Ca and this value decreased with small addition of CaO in the bath.
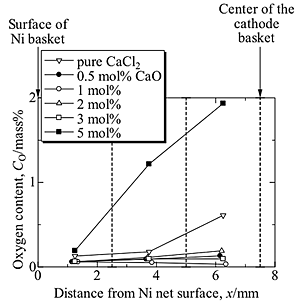
Oxygen distribution in the slightly sintered sample was analyzed with LECO method by separating into 3 parts (Fig.4). Addition of intermediate amount of CaO significantly improves the reduction of NiO. At CaCl2-0.5 mol% CaO for example, the number of NiO peaks detected by XRD measurement decreased as listed in Table 1. The powders containing a single phase of Ni were achieved at runs 3 and 5, where 1.0 and 3.0 mol% CaO were added to CaCl2, respectively. This proved that the reduction was enhanced by the small addition of CaO. Small traces of XRD diffraction peaks due to NiO were detected at the innermost portion of the sintered product when the bath containing 2.0 mol% CaO was used, although the samples obtained from the salt with 1.0 and 3.0 mol% CaO completely transformed to the single phase of Ni.
Oxygen distributions in the sintered sample showed homogeneous trend when 0.5-3.0 mol% CaO was added to CaCl2. When CaO is added in the salt up to 3 mol%, the oxygen contents became smaller compared with the oxygen content when no CaO was added in CaCl2 electrolyte, although electrolysis time was the same. When CaCl2 - 0.5-3.0 mol% CaO was used, more uniform profile in oxygen concentration was achieved, and the residual oxygen at the central part of the sample decreased (Fig.4)
The better reduction and deoxidation at the higher CaO concentration is explained by OS process. When CaO is initially added to the salt, it will decompose and generate larger amount of Ca reductant during electrolysis than that generated when using pure CaCl2. Decomposition and regeneration of CaO will continuously supply Ca reductant for further reduction and deoxidation. The more homogeneous oxygen concentration from the surface to the center of the sintered product can be achieved if the dissolved Ca for NiO reduction was supplied adequately in the central part.
In conclusion, the electrolysis of CaO in CaCl2 molten salt could reduce NiO powder to form metallic Ni. By increasing the concentration of CaO up to 3.0 mol%, the partially sintered pellet with a single phase Ni was obtained with more homogeneous residual oxygen concentration.